
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
For C NMR calculate the chemical shift in ppm for signals 1-7

Transcribed Image Text:1°C-NMR of Unknown E
70
60
50
40
PPM
30
10
20
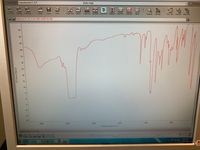
Transcribed Image Text:Experiment: Transmission E.S.P.
(trans.exp)
System
Expl Set Col Bkg Col Smp
Open
Save
Print
Stack Spe Ful Sc
Cmn Scl Aut Bsin Adv ATR Subtract Find Pks Selct All
Clear
Search Lb Mg
Prev Rpt Add NB View NB
Wed Jul 21 16:13:55 2021 (GMT-05:00)
55
50
45
40
35-
30
25
20
15
10
5.
-10
15
-20
3500
3000
2500
2000
1500
1000
Wavenumbers (cm-1)
X (3015.303) Y (-11 794)
4:14 PM
7/21/2021
% Transmittance
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- can the frequency and singlts be found based on the two spectrs for 1HMR and 2CMR and both of them be explainedarrow_forwardIdentify each signal showed in the spectra for both C8H8I2 and C4H9Br. Determine the multiplicity and integrity for each signal.arrow_forwardTrue or false Protons that are not chemically equivalent cannot be magnetically equivalent.arrow_forward
- What is the chemical shift in ppm of a carbon that resonates at 1325 Hz on a 250 MHz instrument (Answer is not 5.3 ppm)?arrow_forwardA proton signal measured in a 90MHz spectrometer shows a doublet with peaks at 5.65 and 5.72 ppm. What is the chemical shift and coupling constant for this doublet? a. δ=5.69ppm, J=5.17 Hz b. δ=5.69ppm, J=6.30 Hz c. δ=5.72ppm, J=6.30 Hz d. δ=5.72ppm, J=5.17 Hzarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY