
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
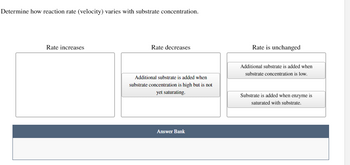
Transcribed Image Text:Determine how reaction rate (velocity) varies with substrate concentration.
Rate increases
Rate decreases
Rate is unchanged
Additional substrate is added when
substrate concentration is high but is not
yet saturating.
Additional substrate is added when
substrate concentration is low.
Substrate is added when enzyme is
saturated with substrate.
Answer Bank
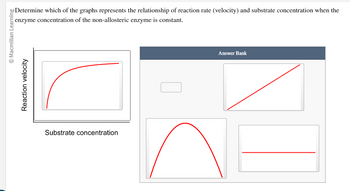
Transcribed Image Text:© Macmillan Learning
Reaction velocity
Determine which of the graphs represents the relationship of reaction rate (velocity) and substrate concentration when the
enzyme concentration of the non-allosteric enzyme is constant.
Substrate concentration
Answer Bank
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Catalysis through proximity and orientation effects involves: (select all that applies) Group of answer choices binding of acidic and/or basic groups in the binding site facilitating chemical reactions by binding substrates close to specific groups binding of substrate in specific, restrictive orientations depending on metal ions for catalysisarrow_forwardCondition: heat What molecule is the enzyme, what molecule is the substrate, and what molecules are the products? Enzyme: Substrate: Product:arrow_forwardDescribe the specific multi-enzyme example discussed in class of how enzyme activity can be altered by changing its substrate to product ratio.arrow_forward
- Order from 1,2,3,4arrow_forwardWhat effect will competitive inhibitor have on the apparent Km of an enzyme for its substrate?arrow_forwardWhich of the following are true when enzymes and substrate interact? kı E + S ● ES- -P k-1 the rate of the formation of ES = k1 [ES] the rate of the formation of the product = k2[ES] k1 [ES] = k-1 [ES] the rate of breakdown of ES to reform the enzyme and substrate = K-1[E][S] the rate of formation of ES = k1[E][S]arrow_forward
- Explain why the maximum initial reaction rate cannot be reached at low substrate concentrations.arrow_forwardAn example of competitive inhibition of an enzyme is step ____ of glycolysis; an example of noncompetitive inhibition of an enzyme is step ______ of glycolysis.arrow_forwardWhat does the Km of an enzyme measure? The affinity (strength of binding) of an enzyme for its substrate. The substrate concentration when AG is 0. The point at which activation energy is overcome. The amount of substrate needed to achieve Vmax- When half of the available enzyme is bound by substrate.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON