Question
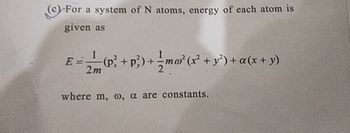
Transcribed Image Text:(c) For a system of N atoms, energy of each atom is
given as
1
E = (P² + p²) + ½ ma² (x² + y²) + a(x + y)
2m
where m, w, a are constants.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- the partition function of an ideal gas of diatomic molecules in an external electric field & is [g(V, T, 8)]" Q(N, V, T, 8) N! where (2mmkT 312 (87 IkT -hv/2kT e q(V,T, 8)= V{ h2 (kT' (µ8 sinh kT) h2 (1 – e-hv/kT) Here I is the moment of inertia of the molecule; v is its fundamental vibrational frequency; and u is its dipole moment. Using this partition function along with the thermodynamic relation, dA = -S dT –p dV – M de where M=Nū, where u is the average dipole moment of a molecule in the direction of the external field &, show that kT] coth kT, Sketch this result versus & from & =0 to & =∞ and interpret it.arrow_forwardAt what temperature would the rms speed of hydrogen atoms equal the following speeds? (Note: The mass of a hydrogen atom is 1.66 x 10-27 kg.) (a) the escape speed from Earth, 1.12 x 104 m/s K (b) the escape speed from the Moon, 2.37 x 10³ m/s Karrow_forwardThe Ritz combination principle can be considered to be a statement of energy conservation. Explain.arrow_forward
- In considering the energy supply for an automobile, the energy per unit mass of the energy source is an important parameter. The "heat of combustion" or stored energy per mass is quite similar for gasoline, ethanol, diesel fuel, cooking oil, methane, and propane. For a broader perspective, compare the energy per mass in joules per kilogram for gasoline, lead-acid batteries, hydrogen, and hay by stating the factor of increase between each one and the next. Hydrogen has "heat of combustion" 142 MJ/kg. For wood, hay, and dry vegetable matter in general, this parameter is 17 MJ/kg. A fully charged 17.0 kg lead-acid battery can deliver power 1450 W for 1.0 hr. (For comparison, the "heat of combustion" of gasoline is about 44 MJ/kg.) battery MJ/kg hay ✕ larger than battery energy/mass gasoline ✕ larger than hay energy/mass hydrogen ✕ larger than gasoline energy/massarrow_forward(4) The scalar product is also useful for applications other than finding the work done by a force. The scalar product makes some problems that would otherwise be difficult relatively easy. Example: Suppose that the data from X-ray diffraction of a crystal indicates that the positions of the atoms in a molecule are as follows: The position of the carbon atom is C = 0i + 0f + 0k, the position of a neighboring oxygen atom is ö = 2î + 2j + k, and a hydrogen atom is located at H = -î + 2î – k. The oxygen atom and the hydrogen atoms are both bonded to the carbon atom. Distances are in nanometers (nm). a. What is the length ||Ō|| of the C-O bond? b. What is the length ||H|| of the C-H bond? c. What is the angle 0 between the C-0 and C-H bonds? You can solve this problem by taking the two ways to find the scalar product and setting them equal to each other, i.e., use the fact that 0 H = OH cos 0 = 0,Hx + 0,Hy+ 0,Hz.arrow_forward(3) The speed of light in vacuum, c, is 3 X 105 km/s and 1 kilometer = 1000 meters. How many Joules of energy are released if 2 kg of matter are converted into energy at 0.1% efficiency? First convert c to units of meters/second: c = 3 x 105 km/s x 1000 m/km = 3 x 108 m/s. Then express efficiency (eff) in decimal terms: eff = 0.001. In this type of problem, the mass/ energy equivalence equation is written as: E = (eff) mc2 Joules,arrow_forward
- Using the same procedure to determine the fundamental equation of chemical thermodynamics (dG = –SdT + VdP) from the Gibbs free energy of a system (G = H – TS), can you please explain how to find the analogous fundamental equation for (A=U-TS)? Also, can you please handwrite the formula down instead of typing? I get confused with typed formulas sometime.arrow_forwardThe gas law for a fixed mass mm of an ideal gas at absolute temperature T, pressure P, and volume V is PV=mRT, where R is the gas constant. Find the partial derivative (∂P/∂V) (∂V/∂T) (∂T/∂P) = ?arrow_forward
arrow_back_ios
arrow_forward_ios