
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
only C

Transcribed Image Text:(a) What is the percentage by mass of NaCl in a saturated solution of sodium chloride at
50°C?
(b) Calculate the solubility of potassium bromide at 23°C. Hint: Assume that the solubility
increases by an equal amount for each degree between 20°C and 30°C.
(c) A saturated solution of barium chloride at 30°C contains 150 g water. How much additional
barium chloride can be dissolved by heating this solution to 60°C?
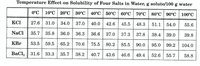
Transcribed Image Text:Temperature Effect on Solubility of Four Salts in Water, g solute/100 g water
0°C
10°C | 20°C | 30°C
40°C
50°C
60°C
70°C
80°C | 90°C 100°C
KCI
27.6 31.0
34.0
37.0
40.0
42.6
45.5
48.3
51.1
54.0
55.6
NaCl
35.7 35.8
36.0
36.3
36.6
37.0
37.3
37.8
38.4
39.0
39.8
KBr
53.5
59.5
65.2
70.6
75.5
80.2
85.5
90.0
95.0
99.2
104.0
BaCl, 31.6 33.3
ВаCl, |
35.7
38.2
40.7
43.6
46.6
49.4
52.6
55.7
58.8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ion Choose an IUPAC name for the structure in 13-a O a. cis-1-isopropyl-2-methylcyclohexane O b. cis-1-propyl-3-methylcyclohexane t of O C. cis-1-ethyl-3-methylcyclohexane d. trans-1-isopropyl-3-methylcyclohexane e. cis-1-isopropyl-3-methylcyclohexane on Choose an IUPAC name for the structure in 13-b a. 3-Benzoicacid nitrile O b. 3-Nitrobenzoic acid of O C. 3-Nitrilebenzoic acid O d. 3-Aminobenzoic acid O e. 3-Cyanobenzoic acid on Choose an IUPAC name for the structure in 13-c a. 1,5-Dimethylheptanoic acid O b. 2,5-Dimethylhexandioic acid of Octanedioic acid O d. 1,5-Dimethylheptandioic acid e. 1,5-Dimethylhexandioic acid O O O Oarrow_forwardNOZ ?. pdarrow_forwardFigure -۹ يفة H A یا اور ان کے C D :0; :2arrow_forward
- Resources Lx Give Up7 Šolution Next Question Question 18 of 25 O My Attempt Y The Newman projection that matches the 3D molecule is: structure and drag to rotate it, or use the controls provided. IC H. CH HO CH H2 H,C. H OH H. HO CH CH, H. H2 H,C но CH3 H2 Zoom In H. Rotate X Rotate Y Rotate Z но CH3 H. Solved MacBook Air fo ofarrow_forwardWhich of the followir i. A studearrow_forward2. Draw the ring flip for the chair structure below. OH CIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY