Question
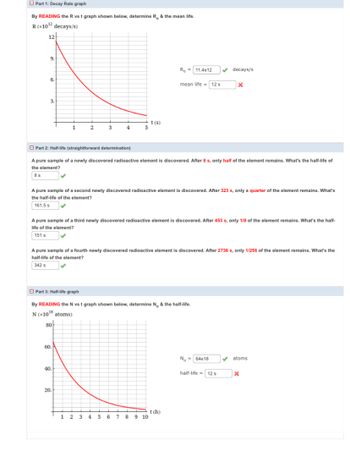
Transcribed Image Text:Part 1: Decay Rate graph
By READING the R vs t graph shown below, determine R. & the mean life.
R (x10¹² decays/s)
12
9.
6.
3.
1
Part 2: Half-life (straightforward determination)
2
60.
40.
3
20.
1
4
A pure sample of a newly discovered radioactive element is discovered. After 8 s, only half of the element remains. What's the half-life of
the element?
8s
A pure sample of a second newly discovered radioactive element is discovered. After 323 s, only a quarter of the element remains. What's
the half-life of the element?
161.5 s ✓
2
t(s)
5
A pure sample of a third newly discovered radioactive element is discovered. After 453 s, only 1/8 of the element remains. What's the half-
life of the element?
151 s
Part 3: Half-life graph
By READING the N vs t graph shown below, determine N. & the half-life.
N (x10¹8 atoms)
80
A pure sample of a fourth newly discovered radioactive element is discovered. After 2736 s, only 1/256 of the element remains. What's the
half-life of the element?
342 s
3 4
Ro 11.4e12
5 6
mean life = 12 s
7 8 9 10
t(h)
decays/s
x
No=64e18
half-life = 12 s
atoms
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Similar questions
- Consider the fission reaction n + 235 U After having found the missing nuclide, calculate the Q-value of the reaction. Enter your result in MeV to 1 decimal place ⁰Xe + ? + 2n. 140-arrow_forward50 Large radionuclides emit an alpha particle rather than other combinations of nucleons because the alpha particle has such a sta- ble, tightly bound structure. To confirm this statement, calculate the disintegration energies for these hypothetical decay processes and discuss the meaning of your findings: (a) 235U – 232Th + ³He, (b) 235U – 23!Th + He, (c) 25U – 230TH + He. The needed atomic masses are 232TH 232.0381 u He 3.0160 u 231'Th 231.0363u 230TH 230.0331 u 235U 235.0429 u "He 4.0026 u SHe 5.0122 uarrow_forwardI need the answer as soon as possiblearrow_forward
- The question is attached below as a image!arrow_forward99Mo beta decays with a half-life of 66 h to 99Tc*, an isotope that is widely used in medical imaging. The 99Mo itself is created in a nuclear reactor by bombarding 235U nuclei with neutrons. One particular 99Mo was created in this way 66 h ago, while another was created just now. Which nucleus is morelikely to decay in the next 66 h?arrow_forwardAnswer letter darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios