
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
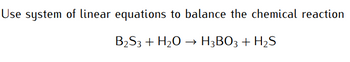
Transcribed Image Text:Use system of linear equations to balance the chemical reaction
B₂S3 + H₂O → H3BO3 + H₂S
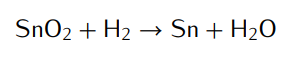
Transcribed Image Text:SnO₂ + H₂ → Sn + H₂O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many water molecules are associated with one formula unit of FeCO3 . H2O? water molecules Write the name of this compound. Name:arrow_forwardWhich atom in the O-C single bond has a partial positive charge (δ⁺)?arrow_forwardDraw the electron distribution diagram for water. Begin with 1 central water molecule. Show the chemistry of each element within the central water molecule (all electron orbits, lone pair electrons, type of chemical bond, polarity/charge, and correct shape). What type of bond creates a water molecule? What type of bond holds 1 water molecule to another water molecule? Next, draw the correct number of other water molecules bonding to the central water molecule. How many other water molecules bond to a central molecule of water?arrow_forward
- Acid Structure and Relative Acidity The strength of an acid, HA, is often determined by the strength and polarity of the H-A bond. In general, a weaker and more polar bond leads to a stronger acid. For binary acids of elements in the same group of the periodic table, the H-A bond strength decreases down the group, so acidity increases. For binary acids of elements in the same row of the periodic table, the polarity of the H-A bond increases from left to right (as the electronegativity of A increases), so acid strength increases. For oxoacids, acid strength increases with the oxidation number of the central atom. If the oxidation number is the same, acid strength increases with the electronegativity of the central atom. Part A Rank the following compounds in order decreasing acid strength using periodic trends. Rank the acid strength from strongest to weakest. To rank items as equivalent, overlap them. ▸ View Available Hint(s) BH₁ HBr HCI H₂S Greatest acid strength The correct ranking…arrow_forwardPart A 13D Explain the term "functional group." How do organic chemists use the functional group concept? A functional group is a group of atoms within a larger molecule that gives the molecule specific physical properties. Organic chemists use functional groups as an organizational tool in classifying, naming, and calculation of the mass of organic molecules. A functional group is an atom within a larger molecule that gives the molecule specific physical and chemical properties. Organic chemists use functional groups as an organizational tool only in classifying and naming of organic molecules. A functional group is a group of atoms within a larger molecule that gives the molecule specific chemical properties. Organic chemists use functional groups as an organizational tool in predicting the properties of organic molecules. A functional group is a group of atoms within a larger molecule that gives the molecule specific physical and chemical properties. Organic chemists use functional…arrow_forwardComplete and balance the equations. Thanksarrow_forward
- Arrange the oxoacids of chlorine according to strength. HCIO, HCIO2,HCIO3,HCIO4arrow_forwardWhich of the following substances would undergo dissociation when placed into a polar solvent such as water? Question 14 options: KBr H2O C6H12O6 CO2arrow_forwardQuestion 24 of 42 Under what conditions do molecules or atoms not intermingle easily at the molecular level? reactants in the solid phase reactants in the gas phase O reactants in the liquid phase O stirring reactants O shaking reactantsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY