
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:<Chapter 10 Problém Set
Exercise 10.76 - Enhanced - with Feedback and Hints
16 of
I Review | Constants | Periodic
MISSED THIS? Read Section
10.9 (Pages 417 - 421) ; Watch KCV 109, IWE 10 10
Submit
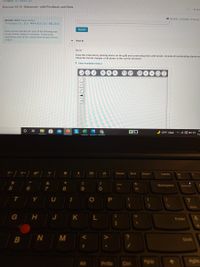
Write a Lewis structure for each of the following ions
Assign formal charges to all atoms. If necessary,
expand the octet on the central atom to lower formal
charge
Part D
BrO2
Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all nonbonding electrons
Show the formal charges of all atoms in the correct structure.
> View Available Hint(s)
L
44%
67°F Clear
へ @D)
11,
*-
Home
End
Insert
Delete
F6
F8
F9
F11
Backspace
NumLock
5
8
9.
Y
U
7
Home
H
JK
4
Enter
B
N M
Shift
立
Transcribed Image Text:2 https://openvellum.ecollege.com/course.html?courseld= 16914186&OpenVellumHMAC=02f20601e6247448103d4b6ce35b6a3de10001
<Chapter 10 Problem Set
Exercise 10.78 - Enhanced - with Feedback
< 17 of 19
MISSED THIS? Read Section 10.9
Review | Constants | Periodic Table
(Pages 417 - 421); Watch KCV 10 9, IWE 10.10
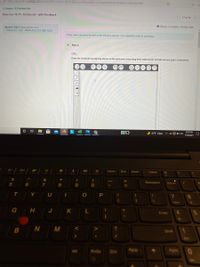
Draw Lewis structures for each of the following species. Use expanded octets as necessary
Part A
CIFS
Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons.
L
44%
67°F Clear A aEO 4)
927 PM
11/6/2021
Home
End
Insert
Delete
FB
F11
Backspace
Numl.ock
6
T.
Y
U
O
7
8.
Home
J
4.
5
Enter
B
N M
1.
Shift
Saut
PriSc
Ciri
Pgup
L.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Helparrow_forwardWhat is the formal charge on chlorine in the best Lewis structure for chloric acid? type your answer...arrow_forward5. Draw all possible Lewis dot structures for the ONNO molecule that satisfy the octet rule (consider only those with the atoms connected as indicated). a) Determine formal charges for each structure. b) Based on the formal charges, rank the structures from least likely to most likely.arrow_forward
- Hydrogen cyanide, HCN, is a highly poisonous compound that vaporizes slightly above room temperature. a) How many valence electrons does C have? b) How many valence electrons does H have? c) How many valence electrons does N have? d) How many total valence electrons does the HCN molecule have? e) Draw the Lewis structure for HCN that minimizes the formal charges on all atoms. f) How many electron domains are on the central atom? g) What is the molecular geometry of HCN? h) Is the molecule polar?arrow_forwardPart A The resulting Lewis structure for NO* has how many shared bonding and unshared nonbonding pairs of electrons? View Available Hint(s) There are 4 shared bonding and 1 unshared nonbonding pairs of electrons. O There are 2 shared bonding and 3 unshared nonbonding pairs of electrons. O There are 1 shared bonding and 4 unshared nonbonding pairs of electrons> O There are 3 shared bonding and 2 unshared nonbonding pairs of electrons. Submit Provide Feedbackarrow_forward2. Which of the following elements can form hyper coordinate compounds? Be, N, Br, F, none of the above can form compounds-with an expanded octetarrow_forward
- 1 What is the total number of valence electrons in the Lewis structure of ICl,"? electrons 2 Draw a Lewis structure for ICl,". • Do not include overall ion charges or formal charges in your drawing. If the species contains oxygen, do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. C opy aste Previous Nextarrow_forwardDraw the Lewis structure of PCl, . Include all the lone pairs. Select Draw Rings Morearrow_forwardWhat would happen to the overall charge on the resulting ion if another hydrogen were to attach to the molecule?arrow_forward
- Create a concept map with "the octet rule" at the center using as many of terms listed below as possible. Use your concept map as an outline to write a paragraph summary. Remember that a concept map should demonstrate your understanding of RELATIONSHIPS between terms by using connecting lines and branches. You are encouraged to add as much personalization as you can or need to; use different colors and connecting words and phrases. Example attached.arrow_forwardClick the "draw structure" button to launch the drawing utility. Write a more stable contributing structure for the following molecule. Be sure to specify formal charges, if any. H₂C I draw structure ...arrow_forwardPlease help me answer all blank if tyoe of electron sharingarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY