
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Propose a synthesis for the compounds below using the reagents given below.

Transcribed Image Text:### Reagents (not necessarily a complete list):
- **PCC**
- **1. OsO₄; 2. KHSO₃**
- **MsCl (or TsCl), pyridine**
- **1. LiAlH₄; 2. H₃O⁺**
- **NaH**
- **SOCl₂**
- **TBDMS-Cl or TMS-Cl, Et₃N**
- **1. RSH, H⁺; 2. Raney Ni**
- **1. Hg(O₂CCF₃)₂, ROH; 2. NaBH₄**
- **POCl₃, pyridine**
- **PBr₃**
- **1. RMgX or RLi; 2. H₃O⁺**
- **NaBH₄**
- **mCPBA**
- **CrO₃, H₂SO₄, H₂O**
- **H⁺, ROH**
- **NaIO₄**
- **HX (X = Halogen)**
- **X₂**
- **1. Hg(OAc)₂, H₂O; 2. NaBH₄**
- **1. H⁺, H₂NNH₂; 2. NaOH**
- **H₂, Lindlar’s catalyst**
- **NaNH₂**
- **1. O₃; 2. Zn, HOAc**
- **1. BH₃-THF; 2. H₂O₂, NaOH**
- **H₂, Pd/C**
- **1. O₃; 2. H₂O**
- **Na, NH₃**
This list includes a variety of reagents commonly used in organic chemistry for different reactions such as oxidation, reduction, protection, and other transformations.
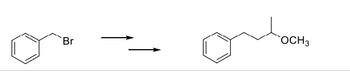
Transcribed Image Text:The image depicts a chemical reaction involving organic compounds.
On the left side, there is a reactant: a benzene ring attached to a bromine atom (C6H5-CH2-Br), commonly referred to as benzyl bromide.
The reaction is shown with a rightward arrow, indicating the transformation of the reactant into a product.
On the right side, the product is shown: a benzene ring attached to a propyl group terminating in a methoxy group (C6H5-CH2-CH(CH3)-OCH3).
This reaction suggests a substitution process where the bromine in benzyl bromide is replaced by a methoxypropyl group.
The diagram does not include specific conditions or reagents necessary for the reaction, such as catalysts, solvents, temperature, or pressure, which are essential for carrying out the substitution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propose a synthesis of the following product from the given starting materials. наб H₂N- НО ОН ОНarrow_forwardhow to synthesize this product?arrow_forwardProvide a synthesis of the target compound from the provided starting material. You may use any additional reagents you need. Clearly separate the reagents used for each step of these multistep syntheses. Br Br но вгarrow_forward
- Complete the reaction schemes below providing the reagents required to achieve thetransformation. More than one step may be necessary for each scheme.arrow_forwardPropose a synthesis for the following compound.arrow_forwardProvide a synthesis of the following compound from the given starting materials. yhan a when wh Me OMe MeO OMe OH Br Me-Iarrow_forward
- Using any necessary reagents, show how the following synthetic transformations may be achieved. Give reagents and conditions for each step. Include all synthetic intermediates (compounds produced during the course of multi-step synthesis). NOTE: You may not use HO CI OH NH₂arrow_forwardProvide the major products for the following reactions?arrow_forward2) Provide a synthesis for the product shown below given the starting material. I ОН ОНarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY