
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%

Transcribed Image Text:When naming disubstituted benzenes, incorporate common names, if possible.
Designate the positions of the groups relative to each other by numbering the groups,
or by using the designations of ortho, meta, or para
Trisubstituted benzenes are named by incorporating common names, if possible. If a
common name is used, that group is automatically given the designation of #1. Give
the lowest numbering possible.
Name each of the following compounds:
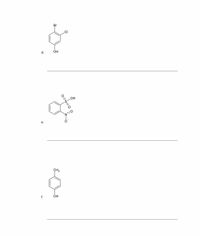
Transcribed Image Text:OH
d.
HO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 47arrow_forwardHow many moles of iron atoms do you have if you have 4.10 x 1023 atoms of iron. (The mass of one mole of iron is 55.85 g.) 1 4 7 +/- 6 ctv A N МacВook Air esc 888 F1 F3 DII DD F4 FB FO @ 23 $ & 1 2 3 4 5 7 8. Q W R T Y A S F H. J K C V M. IG option command MOSISO * 00arrow_forwardName these compoundarrow_forward
- Fictional, not on PT. I found a family of compounds that contain only A, X and Z elements in different multiples of the empirical formula. Using the data contained in the table, find me the molecular formula of a family member with a molar mass of 177.2 g/mol. Use the following table. Element Percent Composition (%) Molar Mass (g/mol) A 40.68 12.01 X 5.08 1 Z ? 16 a. A2X3Z2b. A6X9Z6c. A2XZ2d. A4X2Z3e. A3X6Z3f. AX2Zarrow_forwardOCH3 (cat. H*) HO, b.) OCH3 HO. OCH3 HO но. O O:arrow_forwardНО. OH Br Brarrow_forward
- HBr 1. Li 2. Cul 3. Br OH G AICI 3 Br2 Br2 A B hv tº FeCl3 CH3ONa to NO2 1. BH3, THF (C6H5)3P=CH2 H₂O NaNH2 F E D to 2. H2O2, OH, H₂O HgSO4, H2SO4 Br2 CH2Cl2arrow_forwardCH₂ 1. BH3, THF 2. H₂O₂ ?arrow_forward2. In Alice's Wonderland, every element is different from those in our world. There is a pure unknown compound that consists of element 2 (atomic mass: 22.01 amu) and clement ¥ (atomic mass: 3.13 amu). After elemental analysis of the compound, you discovered that it consists of 70.1 wt. % of clement N and 29.9 wt. % of element V. Answer the following questions. A. What will be the empirical formula of the compound? B. What will be the molecular formula of the compound if the molecular mass of the compound is 94.2 amu?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY