
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
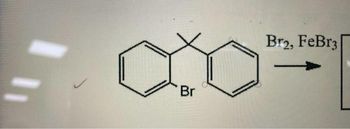
Transcribed Image Text:Br
Br2, FeBr3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chemists use many arrow types to communicate different meanings. Align each arrow type with its purpose. C D Two electron movement 1. A Retrosynthesis 2. B One electron movement 3. С Reaction in equilibrium 4. D 5. Е Irreversible reaction 6. F Resonance >arrow_forwardA colleague produces this graph of the concentration of carbon dioxide as a function of time. In the reaction measured here, is CO, a product or a reactant? Also, in which region is the reaction rate the slowest: A, B, or C? Reaction Involving Carbon Dioxide B Time (min)arrow_forwardA) + CH3-CH2-OH <--H+-->(equilibrium H+) D) + H2O what is the starting product in A and what is ending product in D.arrow_forward
- The reaction diagram shown represents which type of reaction? Reaction Coordinate a. Two-step, slow, exergonic ab. Two-step, fast, endergonic o. One-step, fast, endergonic od. One-step, fast, exergonic e. One-step, slow, exergonic Energyarrow_forwardWhat does it mean if Keg = 1? A. Products are favored over reactants in the reaction. O B. Products and reactants are equally favored in the reaction. C. All of the reactants have turned into products in the reaction. D. Reactants are favored over products in the reaction.arrow_forwardRank the following compounds in order from most able to leave stable, explain your ranking. Use image as referencearrow_forward
- Assign the following reactions as oxidation, reduction or neither. 1. Reaction A [Select] 2. Reaction B 3. Reaction C [Select] To preview the image click here : ÖH B [ Select] : OH PCC CH₂Cl2 conc. H₂SO4/heat H₂, Pd/C :O: darrow_forwardDraw the arrows showing how electron move to get from reactant to product ?arrow_forwardDraw arrows to indicate the movement of electrons in the following reaction. Which direction will this reaction favor? Explain!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY