
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
What would be the proposed steps for the following?
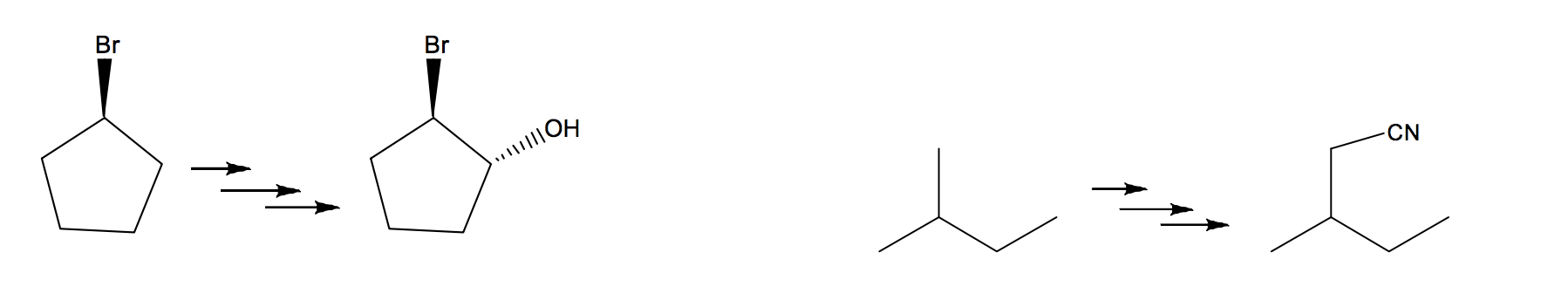
Transcribed Image Text:Br
Br
.ПО
CN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can someone please help me with this questions.arrow_forwardThe synthesis of an azo dye is shown below. Please answer the following questions. NH2 1) Na2CO3 2) NaNO2, HCI B N-Ñ A Š3H ŠO3 Step 1 Step 2 ŠO3 2 1 1. Why you need to add Na2CO3 in step 1? (multiple answers) Oa)To decrease the solubility of compound 1 - "salting out" ob) To deprotonate compound 1, increase solubility in water Oc) To increase the reaction yield Od)To slow down the reaction rate Oe)To neutralize the HCIarrow_forwardWhich of the following scheme is the first step in the following Grignard reaction? + I. BrMg I. BrMg + II. :BrMg IV. :BrMg ※套arrow_forward
- Which sequence of reactions would be the best choice to carry out the following step? ? HO HO 1. MgBr CI, AICI3 1. Br2, FeBr3 2. H30* 2. `MgBr II II CI, AICI3 CI , AICI3 1. 2. Zn(Hg), HCI IV V а. I O b. II c. III O d. IV О е. Varrow_forwardin CHM1450-44635.202240: MyLab X MISSED THIS? Read Section 16.7 (Pages 699 - 701); Watch KCV 16.7, IWE 16.7 Nitrogen dioxide dimerizes according to the following reaction: Course Home ✰ openvellum.ecollege.com/course.html?courseld=17619023&OpenVellumHMAC=cabfcac4e36a69b75c348206dfefb09b#10001 e 2 NO₂ (g) N₂O4 (g) Kp = 6.7 at 298 K A 2.45-L container contains 0.051 mol of NO₂ and 0.081 mol of N₂O4 at 298 K X @ H X G Nitrogen dioxide dimerizes accon X C Consider the following reaction: X Part A Calculate Ke for the reaction. Express the equilibrium constant with respect to concentration to three significant figures. Kc = Submit Part B Submit 17 ΑΣΦ Calculate for the reaction. Express the reaction quotient to three significant figures. N Request Answer P —| ΑΣΦ Request Answer ? P Pearson W Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us | S + ? Review | Constants | Periodic Table X 11:24 PM 10/10/2022arrow_forwardA + Br₂ CH3OH 1.03 2. DMS KMnO4, NaOH cold HI mCPBA 1. OSO4 2. H₂O2 1.03 2. (CH3)2S 17 18 16 15 19 14 CH3CO3H (peroxyacid) B 13 H₂ Pd or Pt (catalyst) HBr 20 1 1. МСРВА 2. H3O+ 12 11 10 2 Pd or Ni (catalyst) D2 (deuterium) 3 4 LO 5 6 7 8 EtOH Br₂ C BH 3 THF H OH H chu chon d ""H OH "OH a H HBr ROOR (peroxide) H₂O H₂SO4 HCI 'OH 1. BH 3 THF 2. H₂O₂, NaOH Br₂ Br₂ H₂O D + H OH a ď™ œ™ OH H ""H OH H HO"arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY