
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
BR - 3
Given the following reaction:
Fe2+ + Cr2O72-→ Fe3+ + Cr3+
Balance the reaction.
The coefficient for water in the balanced reaction is:
a.
none of these
b.
3
c.
7
d.
5
e.
1
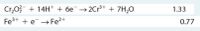
Transcribed Image Text:Cr,03 + 14H* + 6e→2Cr* + 7H,0
Fe3+ + e →Fe?+
1.33
0.77
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the oxidation number of sulphur in the molecule S8? Select one: A. +4 B. +2 C. -2 D. 0arrow_forwardLithium batteries are used throughout our modern world – in cell phones, laptops, tablets, etc. In fact, if you are regularly recharging your device, it’s highly likely it contains a lithium ion battery. The lithium iron phosphate battery provides stable function over time. The overall reaction for the lithium iron phosphate battery is below: LiFePO4 + 6 C -> LiC6 + FePO4 A certain lithium battery weighs 0.6 g and has a capacity of 2250. mA hr (that is, the cell can store charge equivalent to a current of 2250. mA flowing for 1 hr). What is the capacity of this cell in coulombs? What mass of the reactants is needed to deliver 2250. mA hr? What percentage of the cell mass consists of reactants?arrow_forwardSingle 1. As a chemical bond forms between 2 hydrogen atoms in a system, energy is released and the stability of the system Increases Decreases Remains the same 8. In a chemical reaction, as the species is oxidized, its oxidation number Decreases Increases Remains the samearrow_forward
- Please answer all the items and explain/ show your solutions. Will rate if you answer all the items and showed solutions!!!arrow_forward10-please fast The oxidation number for nitrogen in Ba(NO3)2is: Select one: a. +1 b. -3 c. +5 d. +3arrow_forwardplease answer it fastly the oxidizing agent for the following equation is: TiCL4(I) + 2Mg(s) ---> Ti(s) + 2MgCL2 (s) a. MgCL2 b. Mg c. Ti (s) d. TiCL4arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY