
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please answer the following, thank you :)
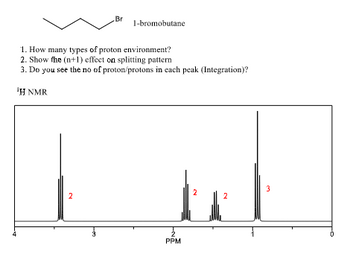
Transcribed Image Text:2
.Br
1. How many types of proton environment?
2. Show the (n+1) effect on splitting pattern
3. Do you see the no of proton/protons in each peak (Integration)?
'H NMR
3
1-bromobutane
2
PPM
2
2
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part A - Reactions of Carbon Dioxide Gas Place a small lump of dry ice into a large test-tube. To the dry ice add about 5 mL of water. After the gas has bubbled through the water for a few minutes (so that it may be assumed the solution is saturated with the gas), test the acidity of the solution by adding 1 drop of universal indicator solution and check the pH on the colour chart provided. Record your observations onto your results page. 1. RESULTS (Remember to not include spectator ions in ionic equations.) Part A - Reactions of Carbon Dioxide Gas 2. Place two large test tubes in the test tube rack. In one tube place a small lump of dry ice and cover the mouth of the tube with a loose plug of cotton wool. Stand the test tube in a beaker of containing a little warm water (from the hot water tap) for a minute. The test tube will fill with carbon dioxide gas. In the meantime, obtain two small (100 or 150 mL) beakers, and place 20 mL of water in each and add 3 drops of universal…arrow_forward16.72 Show how P = kT(a In Q/OV), leads to PV = nRT.arrow_forwardPenicillin F is recovered from a dilute aqueous fermentation broth by extraction with amyl acetate, using 6.5 volumes of solvent per 100. volumes of the aqueous phase. At pH = 3.8 the distribution coefficient KD is 74. (a) What fraction of the penicillin would be recovered in a single ideal stage? (b) What would be the recovery with three-stage extraction using fresh solvent in both stages? (c) How many ideal stages would be needed to give the same recovery as in part (b) if a counterflow cascade were used with V/L = 0.065?arrow_forward
- A spectrometry method was applied to determine component A in solution. The result is shown in the table. Supposing an unknown sample gives result of 525, 536, 529, please calculate the concentration of A in the sample. \table [[C (u),0.00, 1.00, 2.00, 4.00, 8.00, 16.00], [1, 1.2, 110, 216, 445,869, 1750]] A m J c 1) A spectrometry method was applied to determine component A in solution. The result is shown in the table. Supposing an unknown sample gives result of 525, 536, 529, please calculate the concentration of A in the sample. CA (ug/ml) 0.00 1.00 2.00 4.00 8.00 16.00 I 1.2 110 216 445 869 1750arrow_forwardPart (b) Show all work, thank you!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY