
Bobby Drake aka “Iceman” is member of the X-Men who has the physics-defying ability to “manipulate cold and ice” and is depicted lowering the temperature of nearby objects at will. For example, Iceman can freeze an entire pond in seconds just by touching the surface of the water. Let’s put some numbers to this example:
- The pond can be approximated as a hemisphere 20 m in diameter.
- Assume the density of water is 1.00 g cm–3 and independent of temperature.
- The temperature of the pond is initially 17°C and uniform throughout.
- After Iceman freezes the pond, its temperature is –10°C.
a) How many moles of water are in the pond?
b) What is the total amount of heat removed from the pond?
c) Assuming Iceman has a normal human body temperature (37°C), and that his body temperature is constant during the freezing process, what must his change in entropy be (ΔSIceman) so that this process doesn’t violate the 2nd Law of
d) Why isn’t it possible to “manipulate cold” or “increase the coldness” of an object? Explain in 1 or 2 sentences.
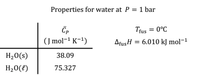
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 5 images

- Calculate the melting point of ice under a pressure of 10 MPa in Kelvin. Assume that the density of ice under these conditions is approximately 0.915g/cm and that of liquid water is 0.998 g/cm?.arrow_forwardShort explanations please.arrow_forwardSelect all statements that apply to constant volume calorimetry. A coffee cup calorimeter is used. A bomb calorimeter is used. The system is the reaction or process. The system is the water plus the calorimeter. The surroundings include the reaction or process only. The surroundings include the water plus the calorimeter. The temperature of the water will always increase. The temperature of the water will always decrease. The temperature of the water could increase or decrease. It is used to measure heat of combustion. It is used to measure heat of solution or reaction.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





