
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Blast furnaces give off many unpleasant and unhealthy gases. If the total air pressure is 1.02 atm, the partial pressure of carbon dioxide is 0.07 ATM, and the partial pressure of hydrogen sulfide is 0.04 atm, what is the partial pressure of the remaining air?
Transcribed Image Text:Tue 12:34 AM Hunte
Luke - Avogadro's Dalto X
Bb normal ideal gas key.pdf
G Four sealed flasks have the sar X
lfcMw4dRuxVM4GIxygmyvOUSJp9eJW9rKulDBYkhIPY/edit#slide=id.gbb7dd383c0_0_72
BIM - 1st
AArt 1-2nd
A WoHistory 4th
A Alg2 - 5th
English - 6th
A Chem - 8th
HAC Grades
a Music
Ideal Gas Laws Practice ☆ D
je Tools Add-ons Help
Last edit was 2 minutes ago
D Present
Background
Layout -
Theme
Transition
1 2 | 3 I 4 l 5. 6 7 8 9
Solve the problem!
Make sure you show your work, circle your answer and Pro AFUSE the correct significant figures!
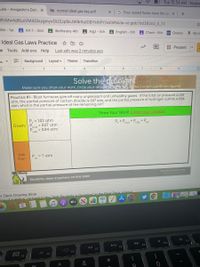
Practice #1 - Blast furnaces give off many unpleasant and unhealthy gases. If the total air pressure is 1.02
atm, the partial pressure of carbon dioxide is 0.07 atm, and the partial pressure of hydrogen sulfide is 0.04
atm, what is the partial pressure of the remaining air?
Show Your Work! Circle your answer!
P = 1.02 atm
Given: P
P = Pco2 + PH2S
+ P
air
= 0.07 atm
CO2
= 0.04 atm
H2S
Ask
For:
P
air
= ? atm
Pear Deck Interactive Slide
De not remove this bar
Students, draw anywhere on this slide!
ar Deck Drawing Slide
upe of question go back to the "Ask Students a Ouestion" in the Pear Deck sidebar
étv
6.
F11
>>
F9
F10
F8
F7
F6
D00
000 F4
*
&
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are the various phases of SiO2?arrow_forwardFind the volume of gas formed upon the decomposition of 1000 g of ammonium perchlorate at 298 K and 1 atm.arrow_forwardIf 2.50 g of zinc metal is reacted with 400 mL of 0.125 mol/L hydrochloric acid. What volume of hydrogen gas would you expect to produce at 30 °C and 110 kPa?arrow_forward
- We're told oxygen is the most common element in the Earth's solid crust. But how much oxygen is there? Let's compare to the amount of oxygen in the Earth's atmosphere, like this: • The most common minerals in the Earth's crust are feldspars, and anorthite (CaAl,Si₂O) is a typical feldspar. Let's assume the entire crust is made of anorthite, with a density of 2.8 g/cm³. • The radius of the Earth is 6371. km and let's say the crust is the upper 75.0 km of it. • Let's model the Earth's atmosphere as a layer on top of the Earth about 100. km thick with an average density of 0.99 g/m³, and which is 23.1% oxygen by mass. Use this information to calculate the ratio of the mass of oxygen in the Earth's atmosphere to the mass of oxygen in the Earth's crust. Round your answer to 2 significant digits.arrow_forwarda sample of dolomitic limestone containing only CaCO3 and MgCO3 was analyzed a) when a 0.2800-gram sample of this limestone was decomposed by heating, 75.o milliliters of CO2 at 750. mm Hg and 20°C were evolved. How many grams of CO2 were produced? b) write equations for the decomposition of both carbonates described above. c) It was also determined that the initial sample contained 0.0448-gram of calcium. What percent of the limestone by mass was CaCO3? d) How many grams of the magnesium-containing product were present in the sample in a. after it had been heated?arrow_forwardWhat is the coordination number (CN) for each type of hole (cubic, octahedral,tetrahedral)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY