
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
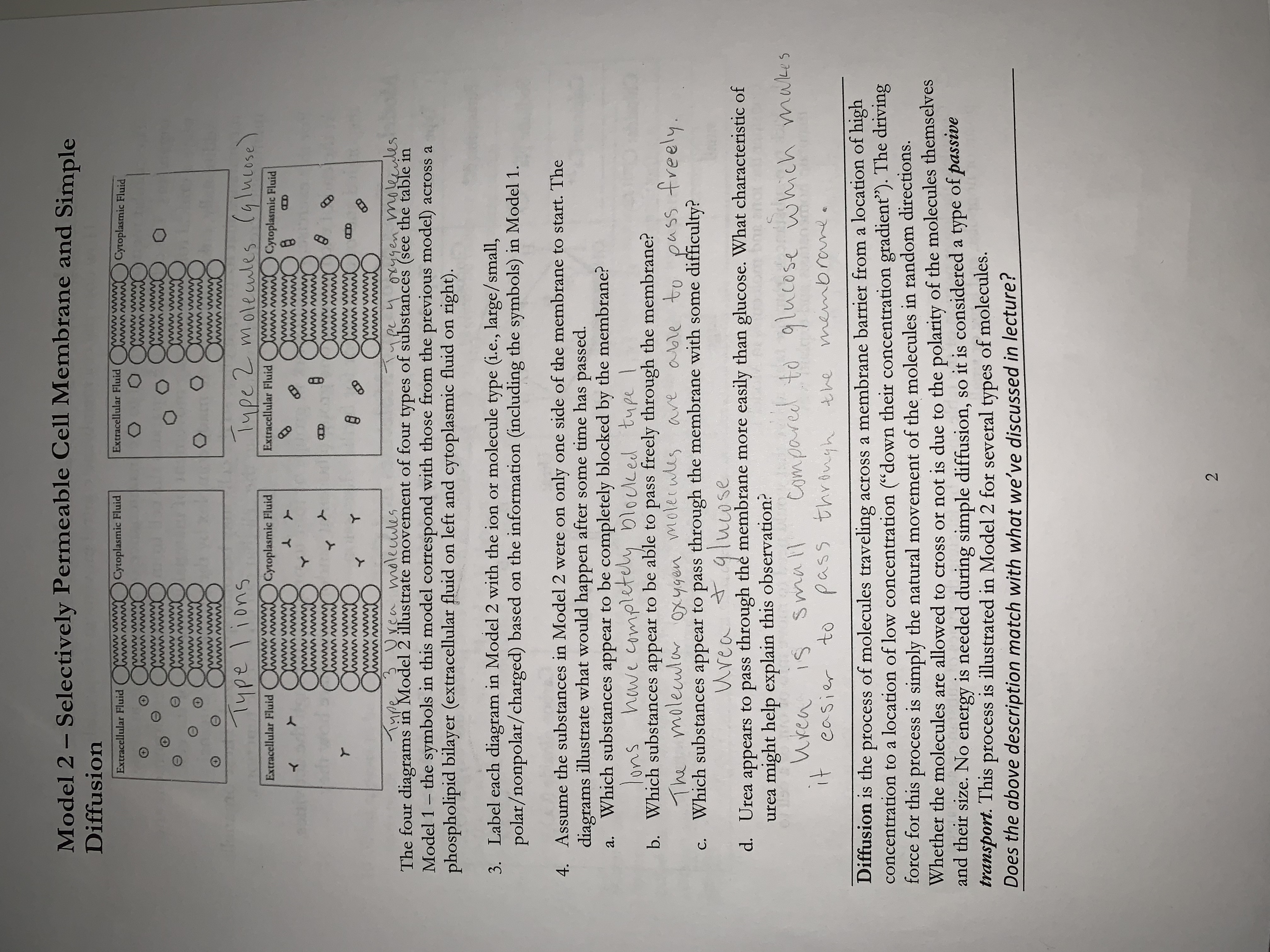
Transcribed Image Text:Model 2 - Selectively Permeable Cell Membrane and Simple
Diffusion
Extracellular Fluid Wwwwww Cytoplasmic Fluid
Extracellular Fluid
www Cytoplasmic Fluid
www
w
wwwwww
www
www.x
www.
www.
www
www
www.
ACTE
Omw
ww
ww
Type lions
Type 2 molecules (glucose)
Extracellular Fluid Wwwwwww Cytoplasmic Fluid
Extracellular Fluid
ww Cytoplasmic Fluid
d
wwwx
w
mo
AM
8
MO
m
un
www
m
8
www
www.x
www.
Summ
Type 3 Urea molecules
upe 4 oxygen molecules
The four diagrams in Model 2 illustrate movement of four types of substances (see the table in
Model 1 - the symbols in this model correspond with those from the previous model) across a
phospholipid bilayer (extracellular fluid on left and cytoplasmic fluid on right).
of
3. Label each diagram in Model 2 with the ion or molecule type (i.e., large/small,
polar/nonpolar/charged) based on the information (including the symbols) in Model 1.
4. Assume the substances in Model 2 were on only one side of the membrane to start. The
diagrams illustrate what would happen after some time has passed.
a.
Which substances appear to be completely blocked by the membrane?
have completely blocked type
lons
b.
Which substances appear to be able to pass freely through the membrane?
The molecular oxygen molecules are
able to pass freely.
c.
Which substances appear to pass through the membrane with some difficulty?
Urea
+
glucose
d.
Urea appears to pass through the membrane more easily than glucose. What characteristic of
urea might help explain this observation?
bon anot
of 115
compared to glucose which makes
potova
urea is
small
easier to pass through the
it
through the membrane
6
Diffusion is the process of molecules traveling across a membrane barrier from a location of high
concentration to a location of low concentration ("down their concentration gradient"). The driving
force for this process is simply the natural movement of the molecules in random directions.
Whether the molecules are allowed to cross or not is due to the polarity of the molecules themselves
and their size. No energy is needed during simple diffusion, so it is considered a type of passive
transport. This process is illustrated in Model 2 for several types of molecules.
Does the above description match with what we've discussed in lecture?
2
CD

Transcribed Image Text:Model 3- Osmosis
Below are three "cells" that are in different aqueous salt solutions (the salt
concentration in the surrounding solution is labeled below each cell). Within each cell,
the salt concentration is ~0.9% NaCl.
OIOIO
0% NaCl
0.9% NaCl
5% NaCl
5. What is an "aqueous" solution?
6.
If the salt ions and membrane behave the same way as illustrated in Model 2 (meaning that these
ions cannot freely cross the membrane), is there any other molecule in this aqueous salt solution
that can cross the membrane?
7. If water could move across the membrane of each of the cells above, then in which direction
would you see net movement of water with respect to each cell? Add arrows to Model 3 to
indicate the net direction of water movement in each scenario.
Osmosis is the process by which a solvent (water) diffuses down its own gradient. If
there is a change in the amount of water in a cell, that has a direct effect on the
volume, shape, and viability of a cell.
8. A hypotonic solution is one in which the solute concentration is lower (hypo-) compared to that
within the cell. Which of the three solutions in Model 3 would you describe as hypotonic?
9. It is dangerous for an animal cell to be placed into a hypotonic solution. Why do you think this
is so? What could happen to an animal cell in a hypotonic solution, based on your response to
#7?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Can these molecules cross into a cell? If yes, with what transport type? Molecule 3 an ion, with an external concentration of 10mM and an internal concentration of 1mM. Molecule 4 a large nonpolar, with an external concentration of 10mM and an internal cellular concentration of 100μM.arrow_forwardell Transport What is shown in the picture below? Plasma membrane C Extracellular fluid -Sodium Na Cytoplasm 11,819 10000 www 0 ATP Phosphate ADP #13 Potassium K* mo 00 20 N Na K" Concentrationarrow_forwardHow is transport across the cell membrane controlled? Diffusion Concentration gradient Permeability What can and can not diffuse through plasma membrane? Factors affecting diffusion: concentration gradient, temperature, molecule size, surface areaarrow_forward
- 5arrow_forwardDraw Your Cells Draw each of your cells here. Cell specialized for diffusion Basic cellarrow_forward1 O2 influx _1 Ca2+ influx 3 K+ influx 2 Na+ efflux 1. simple diffusion _1. Na+ influx by itself 2. facilitated diffusion 1 CO2 efflux 3. primary active transport 2 glucose influx by itself 4. secondary active transport 6 steroid influx or efflux 5. endocytosis 3 Ca2+ efflux that consumes ATP 6. eхосytosis 6 efflux of extracellular proteins 4 Na+ influx that occurs with other substances 2 K+ effluxarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education