
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
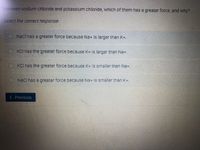
Transcribed Image Text:Between sodium chloride and potassium chloride, which of them has a greater force, and why?
Select the correct response:
NaCl has a greater force because Na+ is larger than K+.
O KCl has the greater force because K+ is larger than Na+.
O KCl has the greater force because K+ is smaller than Na+,
O Načl has a greater force because Na+ is smaller than K+,
( Previous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. Atom 1 Phosphorus Cobalt Germanium Silicon Potassium Cesium Hydrogen Arsenic Lithium Iron Atom 2 Silicon Bromine Selenium Fluorine Nitrogen Oxide Phosphorus Chloride Chloride Carbon Open in DocHub Electronegativity Difference Value Bond Type (Non-polar Covalent, Polar, Ionic)arrow_forwardRank from largest to smallest magnitude in lattice energy To rank items as equivalent, overlap them. ► View Available Hint(s) Largest magnitude BeO KBr KI Csl MgS Reset Help Smallest magnitudearrow_forwardUse the figure to find the electronegativity difference between each of the following pairs of elements, then use the table below to classify the bonds that occur between them as pure covalent, polar covalent, or ionic. (Electronegativity of CrCr is 1.6) Electronegativity Difference (ΔENΔEN) Bond Type Example zero (0−0.40−0.4) pure covalent Cl2Cl2 intermediate (0.4−2.00.4−2.0) polar covalent HFHF large (2.0+2.0+) ionic NaClNaCl Find the electronegativity difference between Br and Br. Express your answer using two significant figures.arrow_forward
- Nitesharrow_forwardThis sketch of a neutral molecule is shaded red or blue wherever the electrostatic potential at the molecule's surface isn't zero. What could the chemical formula of the molecule be? electrostatic potential map tol possible chemical formula (check all that apply) IC1 HBr □ SO3 O HCI □ Br2arrow_forwardGive only typing answer with explanation and conclusion Consider the compounds below. First, identify if the compound is covalent or ionic. If covalent, identify the intermolecular forces that compound will have. Then, rank them based on their melting point – with 1 being highest, 5 being lowest. NaBr LiF H2S C2H6 H2Oarrow_forward
- Select all that apply Which of the following statements correctly describe the Bom-Haber cycle? Select all that apply. The Born-Haber cycle applies Hess's law to a series of hypothetical steps in the formation of an ionic compound. The Born-Haber cycle finds that the overall lattice energy of an lonic compound compensates for the energy needed to form its lons. The Born-Haber cycle applies Hess's law to show thalion formation supplies sufficient energy to form the lattice. The Born-Haber cycle includes lattice energies, which are determined directly tiy experiment. The Born-Haber cycle uses heats of formation, ionization energies, and electron affinities to determine the lattice energy of an ionic compound.arrow_forwardThis sketch of a neutral molecule is shaded red or blue wherever the electrostatic potential at the molecule's surface isn't zero. What could the chemical formula of the molecule be? electrostatic potential map possible chemical formula (check all that apply) HF +ol х N₂H₂ HCN SO₂ SF2 alaarrow_forwardChoose the selection which correctly characterizes all three of the following substances in terms of whether they are polar or nonpolar:CO and SeO2 and CCl4 a) CO is polar and SeO2 is nonpolar and CCl4 is nonpolar. b) CO is nonpolar and SeO2 is polar and CCl4 is nonpolar. c) CO is polar and SeO2 is nonpolar and CCl4 is polar. d) CO is polar and SeO2 is polar and CCl4 is nonpolar. e) CO is polar and SeO2 is polar and CCl4 is polar.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY