
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Please explain and answer the following.
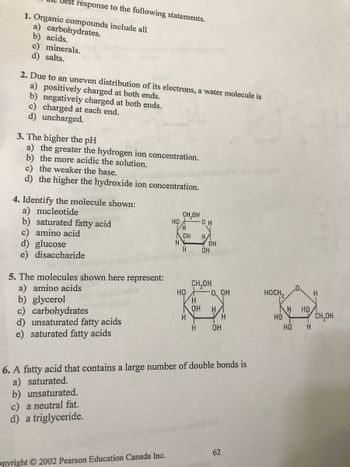
Transcribed Image Text:Best response to the following statements.
1. Organic compounds include all
a) carbohydrates.
b) acids.
c) minerals.
d) salts.
2. Due to an uneven distribution of its electrons, a water molecule is
a) positively charged at both ends.
b) negatively charged at both ends.
c) charged at each end.
d) uncharged.
3. The higher the pH
a) the greater the hydrogen ion concentration.
b) the more acidic the solution.
c) the weaker the base.
d) the higher the hydroxide ion concentration.
4. Identify the molecule shown:
a) nucleotide
b) saturated fatty acid
c) amino acid
d) glucose
e) disaccharide
5. The molecules shown here
a) amino acids
b) glycerol
c) carbohydrates
d) unsaturated fatty acids
e) saturated fatty acids
represent:
c) a neutral fat.
d) a triglyceride.
HO
opyright © 2002 Pearson Education Canada Inc.
H
CH₂OH
H
OH
H
HO
H
0 H
H/
H
OH
ОН
OH
CH₂OH
O, OH
HA
6. A fatty acid that contains a large number of double bonds is
a) saturated.
b) unsaturated.
H
H OH
62
HOCH,
HO
H
HO
НО
H
H
CH₂OH
Expert Solution
arrow_forward
Step 1
1 organic compounds include all
Ans carbohydrates
2 water molecule is uncharged.
3. Higher the pH higher the OH - ion concentration.
4. Molecule is glucose.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can a base such as sodium hydroxide be used to synthesize alum, instead of potassium using hydroxide to synthesize alum? Why or why not?arrow_forwardThe chemical structure of cysteine (C3H-NO₂S) is shown below. Highlight each atom that is in an amino group. :S H | HC-H H-C | H-N-H :0: || C. -0-H X 5 Save For Latearrow_forwardNAME This is an IONIZED amino acid monomer Put a box around the and label the amino group. Does this group function as an ACID or BASE? Put a box around the and label the carboxyl group? Does this group function as an ACID or BASE? Circle the R group. What is this functional group? Use an arrow to show the alpha carbon. What specific amino acid is this? What is the classification of this amino acid? hydrophobic, hydrophilic, Why is it classified that way? U# H CH3 I-Z-I +H-N-C-C. -I H H ΤΑ Redraw this amino acid in the non-ionized form Module Problem 1:38 O O SEAT # charged,arrow_forward
- Synthesis also label any major/minor/trace productsarrow_forwardWhat charged groups are present in lysine at a pH = 7? OA) 1× NH3 B) 1 x COO™ OC) 1× NH3* and 1 x COO- D) 2× NH3 and 1 x COO- OE) 1× NH3+ and 2 x COO- OF) Nonearrow_forwardQ3- We wish to synthesize all possible dipeptides containing the amino acids tyrosine, lysine, phenylalanine, and leucine. Identify the number of possible dipeptides and explain how we would carry this out using combinatorial techniques.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY