
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
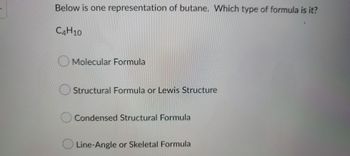
Transcribed Image Text:Below is one representation of butane. Which type of formula is it?
C4H10
Molecular Formula
Structural Formula or Lewis Structure
O Condensed Structural Formula
Line-Angle or Skeletal Formula
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8. Draw the line bond structural formula, the condensed structural formula, and the skeletal structural formula for 2-pentene. Line bond structure: Condensed structure: Skeletal formula: 9. Draw the line bond structural formula, the condensed structural formula, and the skeletal structural formula for 2-methyl-3-heptene. Line bond structure: Condensed structure: Skeletal formula:arrow_forwardWe have thus far determined the single bond connectivity for (CH, ),CO as: H Н—с—с-—с—н H H There are six valence electrons still unaccounted. Carbon must form four bonds in order to satisfy the octet rule. Add a bond to carbon and fill in the remaining missing electrons. Select Draw Rings More H H. H H Harrow_forwardWhat is the condensed structural formula for the following molecule? A. HCO B, CCO C. C2O D. CH3CH2O E C2H5O F. CH3CHO G. C2H4Oarrow_forward
- Draw a skeletal ("line") structure of this molecule: CH3 O CH-CH3 CH3 -C—CH—CH2−C—CH3arrow_forwardI have given you a condensed structure. You need to convert it to an accurate bond-line structure. CH3 CH3-CH-CH2-CH-CH₂-C-H 12-CH-CH2₂-C-1 CI Draw (as bond-line structures) isomers of this compound where you only move the chlorine atom. Draw four isomers of the original compound that would have a five carbon chain as the longest chain. [Note: there would be many isomers that will satisfy this. Find any four.] Using the original molecule (and looking at the carbon next to the aldehyde carbonyl) what would be the charge on that carbon if I removed an H atom and left behind the pair of electrons? Circle the best answer Positive Negative Neutral In the space below, draw that structure (from the sentence above) as a bond-line structure. Then, draw a resonance structure for this ion and be sure to add curved arrows to show the movement of electrons.arrow_forwardCan you please do the last three rows of this table. The trichloroethane, ethane, and bromoethyne?arrow_forward
- Pentane has several isomers. Which of the following condensed formulas is NOT an isomer of pentane? O CH3CH₂C (CH3)2 O CH2(C2H5)2 O CH3CH₂CH₂CH2CH3 O CH3C(CH3)3arrow_forwardBelow is one representation of butane. Which type of formula is it? CH3CH2CH2CH3 Molecular Formula Structural Formula or Lewis Structure O Condensed Structural Formula Line-Angle or Skeletal Formulaarrow_forwardCan you please answer the question #2? Thanksarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY