
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
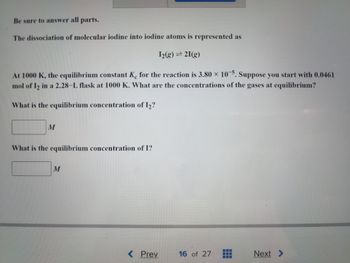
Transcribed Image Text:Be sure to answer all parts.
The dissociation of molecular iodine into iodine atoms is represented as
1₂(g) = 21(g)
At 1000 K, the equilibrium constant Ke for the reaction is 3.80 x 10-5. Suppose you start with 0.0461
mol of 1₂ in a 2.28-L flask at 1000 K. What are the concentrations of the gases at equilibrium?
What is the equilibrium concentration of 1₂?
M
What is the equilibrium concentration of I?
M
< Prev
16 of 27
‒‒‒
‒‒‒
‒‒‒
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The equilibrium constant, K, for the following reaction is 6.59×10-2 at 546 K. PCl5(g) PCl3(g) + Cl2(g) An equilibrium mixture of the three gases in a 6.59 L container at 546 K contains 0.254 M PCl5, 0.129 M PCl3 and 0.129 M Cl2. What will be the concentrations of the three gases once equilibrium has been reestablished, if the volume of the container is increased to 11.7 L? [PCl5] = M [PCl3] = M [Cl2] = Marrow_forward4. For the equilibrium CH₂(g) + 2H₂S(g) = CS₂(g) + 4H₂(g), the concentrations at equilibrium are [CH4] = 0.0804 M, [H₂S] = 0.0608 M, [CS₂] = 0.0196 M, and [H₂] = 0.0784 M at 1400.0 K. Using the equilibrium constant expression from before, calculate Kc.arrow_forwardUsing the general properties of equilibrium constants 0/5 At a certain temperature, the equilibrium constant K for the following reaction is 7.4 × 10 ': N,(e) + 0,(g) -2NO(g) Use this information to complete the following table. Suppose a 35. L reaction vessel is filled with 1.7 mol of NO. What can you say about the composition of the mixture in the vessel at equilibrium? O There will be very little N, and 0,. O There will be very little NO. O Neither of the above is true. What is the equilibrium constant for the following reaction? Round your answer to 2 significant digits. K =] 2 NO(g) N2(0)+O2(a) 1, What is the equilibrium constant for the following reaction? Round your answer to 2 significant digits. K = ] 2N,(0)+20,(0) 4 NO(g) 1,arrow_forward
- A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: -2 N2(g) + 3 H,(g) 2 NH3(g) K,=4. x 10 He fills a reaction vessel at this temperature with 9.0 atm of nitrogen gas and 4.5 atm of hydrogen gas. Use this data to answer the questions in the table below. dla O yes Can you predict the equilibrium pressure of NH, using only the tools available to you within ALEKS? O no If you said yes, then enter the equilibrium pressure of NH, at right. ||atm Round your answer to 1 significant digit. Explanation Check © 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy | Accessibilityarrow_forwardConsider the following equilibrium: 2NOCl (g) <--> 2NO (g) + Cl2 (g) With K = 1.6 x 10-5. 1.00 mole of pure NOCl and 0.964 mole of pure Cl2 are placed in a 1.00 L container. Calculate the equilibrium concentration of Cl2 (g).arrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 NO(g) + Cl₂(g) 2 NOC1 (g) K₂ = 1. x 10 -6 He fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 17. atm of chlorine gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of NOCI, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of NOCI at right. Round your answer to 1 significant digit. O yes O no atm 0 X S BEEN 000 Ararrow_forward
- Suppose a 500. mL flask is filled with 0.20 mol of Br,, 1.6 mol of OCl, and 1.9 mol of BrOCl. The following reaction becomes possible: 2' Br, (g) +OCl, (g)- BROCI(g)+BrC1(g) The equilibrium constantK for this reaction is 5.57 at the temperature of the flask. Calculate the equilibrium molarity of BrOCl. Round your answer to two decimal places. O Marrow_forwardThe equilibrium constant, K. , for the following reaction is 5.10×10-6 at 548 K. NH,CI(s)NH3(g) + HC(g) If an equilibrium mixture of the three compounds in a 5.06 L container at 54S K contains 3.27 mol of NH,Cl(s) and 0.488 mol of NH3, the number of moles of HCI |moles. present isarrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 CH₂(g) C₂H₂(g) + 3H₂(g) K₂=2.x10-5 P He fills a reaction vessel at this temperature with 15. atm of methane gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of C₂H₂, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of C₂H₂ at right. Round your answer to 1 significant digit. yes no atm ☐ x10 X ?arrow_forward
- The equilibrium constant, K, for the following reaction is 3.92×10-2 at 531 K.PCl5(g) PCl3(g) + Cl2(g)An equilibrium mixture of the three gases in a 7.63 L container at 531 K contains 0.331 M PCl5, 0.114 M PCl3 and 0.114 M Cl2. What will be the concentrations of the three gases once equilibrium has been reestablished, if the volume of the container is increased to 18.0 L? [PCl5] = M [PCl3] = M [Cl2] = M Submit Answerarrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 CH₂(g)3H₂(g) + C₂H₂ (g) K₂=6. x 108 He fills a reaction vessel at this temperature with 11. atm of methane gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of H₂, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of H₂ at right. Round your answer to 1 significant digit. yes no at atm x10 Xarrow_forwardAt a certain temperature, the reaction 2HF(g) a H2(3) + F2(g) has K = 1.2 x 10-13. Does this reaction proceed far towards completion when equilibrium is reached? If 0.022 mol HF was placed in a 1.00 L container, and permitted to come to equilibrium, what would be the concentration of H2 and F2 in the container? Concentration of F2 at equilibrium = i M Concentration of H2 at equilibrium = i Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY