
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
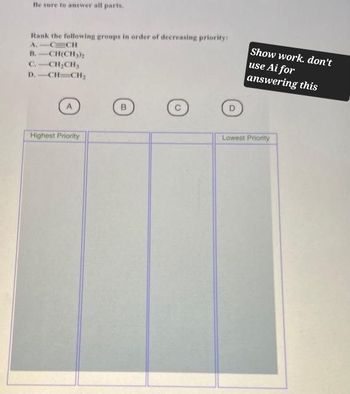
Transcribed Image Text:Be sure to answer all parts.
Rank the following groups in order of decreasing priority:
A.
CCH
B. CH(CH3)2
C.-CH2CH3
D.-CH-CH,
Show work. don't
use Ai for
answering this
Highest Priority
Lowest Priority
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Give the orgamic product: CH₂CH₂CH₂-C=CH I III Select one: O A. III B. II O C. IV OD. I 1) NaNH, 2) CH₂CH₂I -NH₂ II IVarrow_forward2. Determine the best set of reagent(s) necessary to perform the following reaction. a) HBr, ROOR b) xs HBr c) xs Br2, CCl4 d) Br2 (1 eq), CCl4 ? Br Brarrow_forwardSelect all of the molecules that will readily undergo a 1,2-hydride or a 1,2-methyl shift to form a more stable carbocation.arrow_forward
- 6. Used curved arrows to demonstrate mechanisms behind given transformations. Show lone pairs where needed. A. B. C. Br Å Br + Lo Br solo H 05arrow_forwardRedraw the following molecule as a Fischer projection. H3C A. H H CH3 CH3 -Br CI 三 ³0 B. Br- CI CH3 CH3 H CH3 H H C. H- Cl- CH3 CH3 -Br H D. Br H CH3 CH3 -H -CI E. Br CI- CH3 H -H -CH3arrow_forwardWhy are they R and S? Thanksarrow_forward
- For the given molecules, determine if each are in the R or S configuration at the indicated (*) stereo center. Draw each compound and show your work by labelling the priority of each group and using the rotation/swap method as needed.arrow_forwardWhat reagents are needed to convert (CH3CH2)3CC=CH to each compound? a. (CH3CH₂)3CCOCH3 b. (CH3CH₂)3CCH₂CHO C. (CH3CH₂)3CCCl₂CH3 d. (CH3CH₂)3CC=CCH₂CH3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY