
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
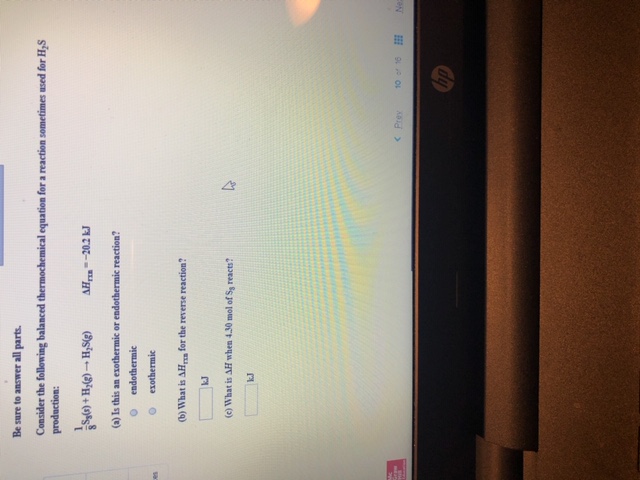
Transcribed Image Text:Be sure to answer all parts.
Consider the following balanced thermochemical equation for a reaction sometimes used for H,S
production:
1
s'H-H+(
(a) Is this an exothermic or endothermic reaction?
AH-20.2 k
endothermic
exothermic
ces
(b) What is AH for the reverse reaction?
kJ
(c) What is AH when 4.30 mol of Ss reacts?
kJ
ra
10 16
<Prey
Ne
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- A mercury mirror forms inside a test tube by the thermal decomposition of mercury(II) oxide. 2 HgO(s) 2 Hg(l) + O2(g) Hrxn = 181.6 kJ (a) How much heat is needed to decompose 546 g of the oxide?kJ(b) If 279 kJ of heat is absorbed, how many grams of mercury form?arrow_forwardThe following thermochemical equation is for the reaction of carbon dioxide(g) with hydrogen(g) to form acetylene(g) (C,H) and water(g). 2C02(g) + 5H2(g)–C,H2(g) + 4H,O(g) AH = 46.5 kJ When 15.3 grams of carbon dioxide(g) react with excess hydrogen(g), kJ of energy are | Hint: An amount of energy is expressed as a positive number. The sign of AH in the thermochemical equation indicates whether the energy is absorbed or evolved.arrow_forwardA Moving to another question will save this response. Question 2 "A new element (Rr) has been synthesized and its properties analyzed. When 0.132g of RrO (s) is added to 166g of water at 24° Cin a coffee cup calorimeter, the following reaction occurs. RrO(s) + H 20(1) – Rr(OH) 2 (aq) AH = -74.8kJ/mol Given the molar mass for RrO is 62g/mol calculate the following moles of Rro round answer to 4 decimal places omit trailing zeroes 9 rxn in joules round answer to the nearest whole number 9 water in joules round answer to the nearest whole number Final temperature round answer to 1 decimal place omit trailing zeroes A Moving to another question will save this response. Chiparrow_forward
- What are products of the following reactionarrow_forwarda) Given that AgNO3 is -124.4 kJ/mol, at standard conditions, what is dH when 56g of AgNO3 (M = 169.9 g/mol) reacts? b) If the heat involved (derived from answer a) is transferred without loss to 100.0g of water at 25ºC, what is the final temperature of the water (CH2O = 4.18J/gºC)?arrow_forwarduestion 27 "A new element (Rr) has been synthesized and its properties analyzed. When 0.157g of RrO (s) is added to 125g of water at 22.2° C in a coffee cup calorimeter, the following reaction occurs. RrO(s) + H 20(1) – Rr(OH) 2 (aq) AH = -83.1k/mol Given the molar mass for RrO is 74g/mol calculate the following moles of Rro round answer to 4 decimal places omit trailing zeroes 9 rxn in joules round answer to the nearest whole number 9 water in joules round answer to the nearest whole number Final temperature round answer to 1 decimal place omit trailing zeroes " A Moving to another question will save this response.arrow_forward
- A certain reaction has Ho = -43.40 kJ and So = 23.20 J/K.(a) Is this reaction exothermic, endothermic or isothermic (neither)?This reaction is________ .(b) Does this reaction lead to a decrease, an increase, or no change in the degree of disorder in the system?This reaction leads to_______ in the disorder of the system.arrow_forward0. Calculate the heat change, in kJ, if 5.9217x1014 ng of phosphorus pentachloride were produced in the following reaction: PC13(g) + C2(g) → PCl:(g) AH°{ = -84.2 kJ/mol %3Darrow_forwardWhat is the kinetic energy of a 4.15x10 kilogram golf ball moving at a velocity of 44.3 meters per second? (KE =½m v) Joulesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY