
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:vork
Saved
attempts left
Check my work
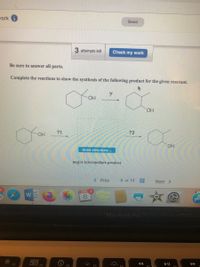
Be sure to answer all parts.
Complete the reactions to show the synthesis of the following product for the given reactant.
HO.
OH
?1
?2
OH
draw structure ..
major intermediate product
< Prev
3 of 14
Next >
MAR 2
W
8.
MacBook Pro
F2
F4
F5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6. Provide the product for each of the following reaction. Indicate the type of reaction based on the reaction conditions. Show the complete mechanism for each reaction. Show the energy diagram for the reaction. F3C. CH3 CH3OH 25 °Carrow_forwardHow does gold help to catalyze the reaction in the gold catalysis experiment? It deprotonates the methanol, making methanol a better nucleophile. оа. o b. It donates electron density to the alkyne, making the alkyne a better nucleophile. О с. It coordinates to the methanol, making methanol a better nucleophile. o d. It coordinates to the alkyne, making the alkyne a better electrophile.arrow_forward← Draw the major product of this reaction. Ignore inorganic byproducts. H 1. NaBH4 2. Neutralizing work-uparrow_forward
- Hi, it's one question please show all the work clearlyarrow_forward4. Draw the final major product(s) for each multistep synthesis reaction. H3O* OMe H3PO4 Jones Et,NH reagent H30* PBR3 KOC(CH3)3 1. O3 NABH3CN HO- 2. DMS NH3, H3O* Br KOC(CH3)3 1. ВНз PCC H3O* 2. H2О2, ОН CH2CI2arrow_forwardQ3. Give the major organic product(s) of each of the following reactions or sequences of reactions. 1. CH₂ OH Bra CH₂COOH 1. Bra PBts 2. H₂Oarrow_forward
- Which best explains the following reaction. OH OH H3O+ This reaction does not work because the major product would be an alkene formed by elimination. This reaction does not work because the major product would be an ester. This reaction does not work because three ethers can be formed. This reaction works and the only ether product formed is shown. +arrow_forwardWhat is the major product of the following reaction? Use a line structure which means that you should not draw in H atoms and should not enter C for carbons unless necessary. Convert your answer to the InChl format and enter it as your answer. OH H3PO4, A Major product Minor product 고 고arrow_forwardHO .OH cat. H2SO4 H₂O cat. H2SO4 Complete both mechanisms above. BRIEFLY explain how the different reaction conditions (in the presence of acid) result in different products being formed. How does equilibrium play a role?arrow_forward
- 29 minutes, 42 seconds. Question Completion Status: A Moving to another question will save this response. Question 15 What is not an expected product of the following allylic substitution reaction? NBS, hv Br Br Compounds II and II Compound II only O Compound I only O Compound II only A Moving to another question will save this response O O Carrow_forwardGive the complete arrow pushing mechanism to show the formation of the product in the given reaction.arrow_forwardFor each reaction shown below, draw the major organic product that would form. 1) Br NO2 ONa NO2 CI 2) NO2 *KS- *ks->-OMe OLi O₂N. Br LiO 3) O₂N Br high dilution Ο Ν NO2 1/2 eq. Br LiO OLiarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY