
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
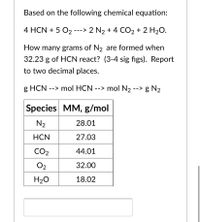
Transcribed Image Text:Based on the following chemical equation:
4 HCN + 5 O2 ---> 2 N2 + 4 CO2 + 2 H2O.
How many grams of N2 are formed when
32.23 g of HCN react? (3-4 sig figs). Report
to two decimal places.
g HCN --> mol HCN --> mol N2 --> g N2
Species MM, g/mol
N2
28.01
HCN
27.03
CO2
44.01
O2
32.00
H20
18.02
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How do I solve?arrow_forward4.Given the balanced chemical equation below, determine the mass of AgCl produced when 11g of Silver nitrate reacts with 15g of Sodium chloride. AgNO3 + NaCl --> AgCl + NaNO3 _________g AgCl How many grams of excess reactant remain after the reaction_________ g Blank 1: Blank 2: show all workarrow_forwardB. Stoichiometry and Percent Yield DATA 1. Mass of sodium carbonate 2. Mass of filter paper 3. Mass of dry filter paper and calcium carbonate product CALCULATIONS AND QUESTIONS (show all of your work) 1. Mass of dried product (actual yield) (Mass of dry filter paper and calcium carbonate product - mass of filter paper) 1.0 g 1.2g 2.69 2. Write the balanced chemical equation for the reaction of sodium carbonate and calcium chloride. (Ignore the hydrate in this equation) 2.89 3. Use stoichiometry to calculate the theoretical yield of calcium carbonate product based on the starting mass of sodium carbonate. 4. Calculate the percent yield of the reaction based on the actual yield of dried calcium carbonate product and the theoretical yield of calcium carbonate product. 11arrow_forward
- Visited Use the References to access important values if needed for this question. According to the following reaction, how many grams of iron(III) chloride will be formed upon the complete reaction of 22.1 grams of chlorine gas with excess iron? 2Fe(s) + 3Cl₂(g) → 2FeClę (s) giron(III) chloride Submit Answer Q Search H N & 81 U 18 * M Retry Entire Group 29. • C fo K hp 9 more group attempts remaining no 111 O 12-->> [ ** ins W prt sc. Previous (NEW) delete backspace home lock enter 1 o 5:35 PM 5/14/2023 end 7 Varrow_forwardFor the following balanced equation: CH4 + 4 Cl2 ==> CCl4 + 4 HCl Determine the number of moles of HCl that can be made from 6.80 moles of CH4? Answer to 2 decimal places.arrow_forward2911/15/-1 {J... macOS Monterey... Combining 0.287 mol Fe₂O3 with excess carbon produced 13.5 g Fe. Fe₂O3 + 3C 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: What is the theoretical yield of iron in moles? theoretical yield: Dashboard scyool What is the percent yield? percent yield: Resources mol mol %arrow_forward
- 2 KMN0, + 16 HCi → 2 KCI + 2 MnCl, + 8 H,0+ 5 Cl, a. How many grams of KMN0, are needed to produce with 1.2 moles of KCl? b. How many many moles of Cl, are produced from 3.1 grams of HCl?arrow_forwardWhen calcium carbonate is added to hydrochloric acid, calcium chloride,carbon dioxide, and water are produced. how many grams of the excess reactant will remain after the reaction is complete? Mass of excess reactant:_____garrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY