
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
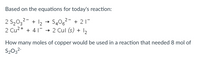
Transcribed Image Text:Based on the equations for today's reaction:
2 S2032- + 12 + S406²- + 21-
2 Cu2+ + 41
2 Cul (s) + I2
How many moles of copper would be used in a reaction that needed 8 mol of
S2032-
Expert Solution
arrow_forward
Step 1: Given data of the problem
For the given reaction:
2S2O32- + I2 S4O62- + 2I-
2Cu2+ + 4I- 2Cul (s) + I2
We have to find moles of copper ion used for 8 mol of S2O32- .
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance the chemical equation. What is the coefficient in front of O₂? C3H8(1) + ? O2(g) →→→ CO2(g) + H₂O(g) 02 3 O4 05 none of thesearrow_forwardComplete and balance the following reaction, then give the product side of the balanced equation. __ Cu (s) + __ ZnSO4 →arrow_forwardBalance the following chemical equation: B2Br6+B2Br6+HNO3→HNO3→B(NO3)3+B(NO3)3+HBrarrow_forward
- complete the balanced molecular chemical equation for the reaction below. if no reaction occurs, write NR after the reaction arrow KOH(aq)+AlCl3(aq)=arrow_forwardIdentify the type of chemical equations and balance the given equations below. Fe + CO2 Al + H2SO4 → Al2(SO4)3 + H2 FeO + C →arrow_forwardClassifying Reactions and Balancing Chemical Equations Part A: For each of the chemical reactions that are listed below, balance the chemical equation and then state the type of chemical reaction. 1. - Cu (s) + O₂(g) → CuO (s) Reaction type: 2.H₂O ()→→O₂ (g) + H₂ (B) Reaction type: 3. Fe (s) + H₂O () Fe₂O3 (s) + H₂(g) Reaction type: 5. 4.H₂S (aq) + AsCl3 (aq) →→As2S3 (s) + HCl(aq) Reaction type: CaCO3 (s) → CO₂(g) + Reaction type: 6. C4H10O (g) + O₂(g) →→CO₂(g) + H₂O (c) Reaction type: 7. S8 (s) + Fe (s)→ FeS (s) Reaction type: CaO (s) 8. H₂SO4 (aq) +Al (s) →→Al2(SO4)3 (aq) + H₂(g) Reaction type: 9. H3PO4 (aq) + NH,OH (aq) Reaction type: (NH4)3PO4 (aq) + stei HOH (c) 2017-arrow_forward
- Complete (where necessary) and balance the following equation. Write the stoichiometric equation, then write the equation in ionic and net ionic form. __ Cu(OH)2 (s) → CuO (s) + __________arrow_forwardA chemist goes into the lab and performs an experiment in which a mixture of 8.47 grams of Zn and 6.03 g of sulfur are reacted. Before starting the experiment they perform the following calculations to predict the maximum (theoretical) yield of zinc sulfide that could be produced. First, write a balanced equation: Zn + 2 S → ZnS2 Second, check to see which reagent is limiting: 1 mole Zn 1 mole ZnS, 1 mole Zn 129.4 g ZnS, 1 mole ZnS, 8.47 g Zn × = 16.8 g ZnS, %3D 65.4 g Zn 1 mole ZnS, 129.4 g ZnS, 1 mole ZnS, 1 mole S 6.03 g S × = 12.2 g ZnS, 32.0 g S 2 moles S Since 6.03 g S produces the least amount of ZnS2, the S is limiting! They obtain an actual yield of 8.25 grams of zinc sulfide. Calculate their percent yield to one decimal place.arrow_forwardChoose the correct chemical equation for a combustion reaction. O 2C;Hs + 1102 → 6CO2 + 6H20 O 2C3 H6 + 902 → 6CO2 + 6H2O O 2C;H6 + 902 6CO + 6H2 o O 2C;H6 + 702 → 6CO2 + 6H20arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY