
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Based on the data represented in the temperature vs volume plots, was the simple distillation or the fractional distillation more effective for separating the mixture of 2-Butanone and toluene.Explain how the data in the graph supports your answer
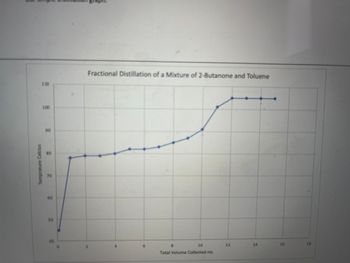
Transcribed Image Text:Temprature Celcius
110
100
90
BO
70
60
50
40
Fractional Distillation of a Mixture of 2-Butanone and Toluene
2
8
10
Total Volume Collected mL
12
14
16
18
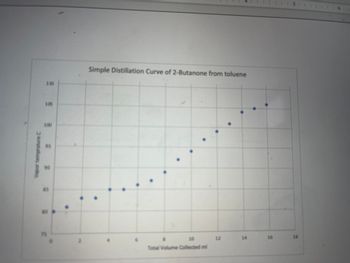
Transcribed Image Text:Vapor temprature C
110
105
1.00
95
90
85
80
75
Simple Distillation Curve of 2-Butanone from toluene
Total Volume Collected mi
12
14
16
151111
18
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why did you measure 60 ml and not 59.04 ml to make up this final solution?arrow_forwardThe experimental data in the table was collected during a freezing point depression study where BHT (butylated hydroxytoluene) was the solvent. Mass of BHT Mass of unknown Freezing point of pure BHT Freezing point of BHT and unknown solution Kf for BHT 7.709 g7.709 g 1.252 g1.252 g 74.17 ∘C74.17 ∘C 70.91 ∘C70.91 ∘C 6.83 ∘C/?6.83 ∘C/m Use this data to calculate the molar mass of the unknown solute. molar mass =arrow_forwardshow full and complete procedure HANDWRITTEN onlyarrow_forward
- Define density. Hexane has a density of 0.659 g/mL. How many milliliters (mL) of hexane are in 15.0 grams (g) of hexane?arrow_forwardWhen performing arecrystallization, slocent with a boiling point less must be use than the melting point of the compound being recrystallized. For benzil ( m.p.94-95C) which solvent would be best for this recrystallization? toluene (b.p. 110C) H2O (b.p.100 C) ethane( bo -98) methanol(bp64.7)arrow_forwardConsider 15 mL of an aqueous solution containing 2.0 g of an organic solute. If the distribution coefficient for the solute between ether and water is 20, calculate the percent of the compound that can be recovered with a single extraction with 13.3 mL of ether. to two significant figures.arrow_forward
- Your compound exhibits the following solubility behavior (S = soluble, P partially soluble, I = insoluble). Whic solvent is the best choice for recrystallization? Solvent water heptane diethyl ether decane dichloromethane b.p (°C) 100 98 35 174 40 hot solvent 1. cold solvent O decane heptane )dichloromethane water diethyl etherarrow_forwardTrue/Falsearrow_forwardHow is it that methyl alcohol is soluble with water but not hexane?arrow_forward
- In a mixed solvent system, what is the role of the "poor" solvent? a.to DECREASE the solubility of the solute in the good solvent and saturate the solution b. to INCREASE the solubility of the solute in the good solvent and saturate the solution c. to DECREASE the boiling point of the mixturearrow_forwardName: Date: Section: Prelaboratory Assignment: Determination of Molecular Weight Through Freezing Point Depression Analysis 1. What is the vapor pressure at 20'C of a solution of 3.50 g of naphthalene C1Hg in 25.0 g of benzene (v.p. pure benzene =74.7 mm Hg at 20°C) 2. Calculate the freezing point of a solution of 25 mL of CH,OH (d = 0.792 g/ml) in 325 mL of water (d= 1.00 g/ml, k, 1.86 °C/m). %3D %3D %3D A5.00arrow_forward6. During the recrystallization procedure, a two-solvent system is used. What is a two-solvent system. Explain why two-solvent systems are used in recrystallization.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY