
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Based on the calculated Δ?vap values, arrange the liquid samples in order from most ordered to least ordered.
CH3OH
C6H6
C4H10

Transcribed Image Text:### Heat of Vaporization and Entropy of Vaporization
Three liquid samples of known masses are heated to their boiling points using a heater rated at 525.0 W. Once the boiling points of each sample are reached, the samples are heated for an additional 3.60 minutes, which results in the vaporization of some of each sample. After 3.60 minutes, the samples are cooled and the masses of the remaining liquids are determined. The process is performed at constant pressure. The results are recorded in the table below.
| Liquid | Boiling point (°C) | Initial mass (g) | Final mass (g) |
|-----------|--------------------|------------------|----------------|
| CH<sub>3</sub>OH | 64.5 | 407.65 | 312.53 |
| C<sub>4</sub>H<sub>10</sub> | -1.00 | 410.2 | 105.08 |
| C<sub>6</sub>H<sub>6</sub> | 80.1 | 593.3 | 331.18 |
**Objective:**
Calculate the molar enthalpy of vaporization, ΔH<sub>vap</sub>, and the molar entropy of vaporization, ΔS<sub>vap</sub>, for each sample. Assume that all of the heat from the heater goes into the sample.
**Calculations:**
1. **ΔH<sub>vap</sub> of CH<sub>3</sub>OH**:
- Input box for calculation result (kJ/mol)
2. **ΔS<sub>vap</sub> of CH<sub>3</sub>OH**:
- Input box for calculation result (J/mol·K)
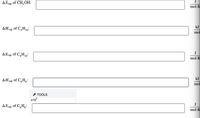
Transcribed Image Text:### Thermodynamic Properties of Various Compounds
In the context of phase changes, particularly vaporization, we often examine the enthalpy (\( \Delta H \)) and entropy (\( \Delta S \)) of vaporization. Below is a table format into which data for the various compounds can be filled.
#### Methanol (\( \text{CH}_3\text{OH} \))
- **Entropy of Vaporization (\( \Delta S_{\text{vap}} \))** of \( \text{CH}_3\text{OH} \)):
- Input your value here: `_________________` J/mol·K
#### Butane (\( \text{C}_4\text{H}_{10} \))
- **Enthalpy of Vaporization (\( \Delta H_{\text{vap}} \))** of \( \text{C}_4\text{H}_{10} \)):
- Input your value here: `_________________` kJ/mol
- **Entropy of Vaporization (\( \Delta S_{\text{vap}} \))** of \( \text{C}_4\text{H}_{10} \)):
- Input your value here: `_________________` J/mol·K
#### Benzene (\( \text{C}_6\text{H}_6 \))
- **Enthalpy of Vaporization (\( \Delta H_{\text{vap}} \))** of \( \text{C}_6\text{H}_6 \)):
- Input your value here: `_________________` kJ/mol
- **Entropy of Vaporization (\( \Delta S_{\text{vap}} \))** of \( \text{C}_6\text{H}_6 \)):
- Input your value here: `_________________` J/mol·K
### Measurement Units
- **Enthalpy of vaporization (\( \Delta H_{\text{vap}} \))** is measured in kilojoules per mole (kJ/mol).
- **Entropy of vaporization (\( \Delta S_{\text{vap}} \))** is measured in joules per mole per kelvin (J/mol·K).
These values are essential for understanding the energy requirements and changes associated with phase transitions from liquid to gas for these compounds.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Find the boiling temperature at 760 torr of an isomer of octane, C3H18, if its enthalpy of vaporization is 38,210 J mol-1 and its vapor pressure at 110.o°C is 638.43 torr. A 118.30°C 111.52°C B 113.22°C 115.00°C D 115.65°Carrow_forwardAt 25.0°C, the vapor pressure of butane is 2.30 atm. What is the pressure in the container at 130.0°C? (ΔH° vap = 24.3 kJ/mol.)arrow_forward7. The vapor pressure of isooctane is 75.0 torr at 34.0 °C. What is the normal boiling point Given: Heat of vaporization of isooctane = 33.8 kJ/molarrow_forward
- The vapor pressure, P, of a certain liquid was measured at two different temperatures, T. The data is shown in this table. T(K) P (torr) 308.9 147.3 3514 785.8 If you were going to graphically determine the enthalpy of vaporization, AH for this liquid, what points would you plot? To avoid rounding errors, use three significant figures in the x values and four significant figures in the y values. point 1: x- point I: y- point 2: x- point 2: y- Determine the rise, run, and slope of the line formed by these points. rise: run: slope: What is the enthalpy of vaporization for this liquid? All J/molarrow_forwardThe vapor pressure of ethanol (C2H5OH) at 19 °C is 40.0torr. A 1.00-g sample of ethanol is placed in a 2.00 L containerat 19 °C. If the container is closed and the ethanolis allowed to reach equilibrium with its vapor, how manygrams of liquid ethanol remain?arrow_forwardA chemist determined vapor pressures of an unknown substance at different temperatures. The data was plotted as ln(P) vs 1/T (temperature in Kelvin). What is △H∘vap of this substance in kJ/mol?arrow_forward
- please explainarrow_forwardLabelling a typical simple phase diagram.arrow_forwardEthanol, with AHvap = 40.5 kJ mol, has a higher AHvap than methanol, which is 35.2 kJ mol, due to its larger size and greater intermolecular forces. Because the AH for ethanol is larger, we would expect that for the two liquids to reach the same vapor pressure, the temperature for ethanol would have to be higher. If the vapor pressure is 0.0992 atm at 27.3 °C, what would the temperature be when the vapor pressure is 4.87 atm? The temperature will be K. e Textbook and Mediaarrow_forward
- Nonearrow_forwardEthanol, with ΔHvap = 40.5 kJ mol-1, has a higher ΔHvap than methanol, which is 35.2 kJ mol-1, due to its larger size and greater intermolecular forces. Because the ΔHvap for ethanol is larger, we would expect that for the two liquids to reach the same vapor pressure, the temperature for ethanol would have to be higher. If the vapor pressure is 0.0992 atm at 27.3 °C, what would the temperature be when the vapor pressure is 4.52 atm?arrow_forwardMeasurements of the saturated vapor pressure of liquid SiCl4 give 0.1396 atm at 280 K and 0.4880 atm at 310 K. Calculate the normal boiling temperature of SiC14.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY