
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Balence the equation
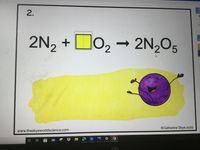
Transcribed Image Text:2.
2N, +Do, - 2N,Og
O2→
©Catherine Skye 2020
www.theskyeworldscience.com
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student combined Ba (s) and HCl (aq). The student observed bubbling, thus a reaction occurred. 1. Write the balanced molecular equation for this reaction showing coefficients and phase labels. 2. Write the complete ionic equation for this reaction. 3. Write the net ionic equation for this reaction.arrow_forwardWhat is a calibration curve? (select the BEST description) A calibration curve is a serial dilution. O a. O b. A calibration curve is a graph that gives information. O c. A calibration curve is a method for determining the concentration of an unknown sample by comparing the unknown to a set of standard samples of known concentration. When using FerroZineM to determine the concentration of iron, how many moles of FerroZineTM are needed to complex 3.6x107 mol of iron? Answer:arrow_forwardQuestion 14arrow_forward
- IV (intravenous) solutions are mixtures of sugars, salts and other aqueous solutes fed directly to a patient's veins. If an IV solution is 5.0% glucose by mass, how many grams of solid glucose would be require to prepare 60.0 milliliters (ml) of the solution? Assume the density of the solution is 1.0 g/mlarrow_forwardWhat can not be changed when balancing equations? A. subscripts B. the ratio of compounds C. the number in front of compounds D. none of the above.arrow_forwardThe energy changes in chemical reactions occurs when atoms _____ to form new substances. dissociate enlarge rearrangearrow_forward
- Write the total ionic equation for the decomposition of a solid of zinc chlorate when is heated. Note: you need to predict the products first.arrow_forwardGive typed full explanationarrow_forwardBe sure to answer all parts. How many H atoms are in 84.9 g of isopropanol (rubbing alcohol), C,H,0? Enter your answer in scientific notation. x 10 H atomsarrow_forward
- Will potassium permanganate be soluble in water? Explain your answer. If your answer to the first question is yes, then write the dissociation reaction for potassium permanganate.arrow_forwardhow is changing the color of a liquid to change the temperature related to chemical reactionarrow_forwardI'm not quite sure how to do thisarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY