
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:C Balance the thre X
ps://app.101edu.co
cions.pdf
y
1
FI
2
11
W
S
C In this experime X
F2
3
E
D
Lab #7 Redox Tis
F3
$
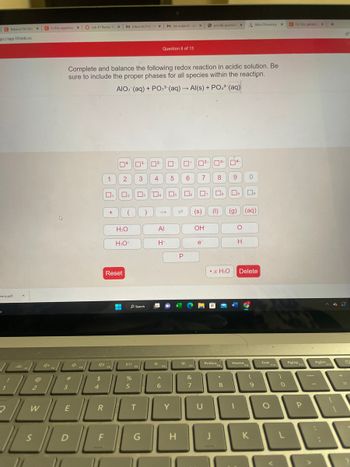
Complete and balance the following redox reaction in acidic solution. Be
sure to include the proper phases for all species within the reaction.
AIO: (aq) + PO3³ (aq) → Al(s) + PO4³ (aq)
4
R
F
1
+
X
F4
M Inbox (4,311)-b x
4
Reset
2 3
H₂O
H3O+
0³-0²-
DII
( ) -
Al
%
5
02 03 04 05 06 0:
07
Search
FS
4
T
G
*
Question 6 of 13
M (no subject) -sc X
0
H+
A
6
F6
Y
☐
5 6 7 8
H
P
= (s) (1)
*
&
7
pre lab question X
OH
2+ 3+
e
U
PrtScn
SCF8
9
8
☐ ☐o
(g) (aq)
O
• x H₂O Delete
.
H
I
0
Home
Aktiv Chemistry X
F9
(
9
K
End
O
F10
)
0
L
C For the galvanic X
PgUP 11
x +
P
PgDn
A
:
F12
L
(
=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. NO2(g) → NO(aq) 1 2 + 4- 3_ 2. ப + 2+ 3+ ल 4+ 3 4 LO 5 CO 6 7 8 9 0 ☐ 3 ☐ 4 ☐ 5 ☐ 6 6 07 口。 وا 口。 ☐ 2 () H +H Reset (s) N О OH e (I) x H₂O (g) (aq) H₂O+ 3 H₂O 2 Deletearrow_forwardFor the following redox reactions, please balance them in basic solution and indicate the impact of pH on the reaction (e.g., lower pH favors, disfavors, has no effect on the reaction).arrow_forwardPlease Show reaekson and don't use hend raitingarrow_forward
- Name: 1. Balance the following redox reaction in an acidic and a basic solution: Hg(1) + H₂SO4(aq) + Cr₂O²(aq) → Hg2SO4(s) + Cr³+ (aq) 3+ Isai bas ishini srl sbrian NetID: oilaser oss ni hoorhon brus bssibixo al tadi esiboga si dra antst (pp) 500S + (pm) 1(ps) H (1)O,HY + (pa) med + (pm) *² + (pm) *25+- (p) Ter (ps) D robes griwallot orii noi (engrarlo bine stiam to estale diw stolqmos) mistgrib flesarrow_forwardPlease don't provide handwritten solution .....arrow_forward(1) Identify each of the following half-reactions as either an 3. Combini... 2req oxidation half-reaction or a reduction half-reaction. Question Question half-reaction identification Question 4. Full Re... 2req H2(g)2H*(aq) + 2e¯ 5. Reducti... 2req |Cl2(g) + 2e →2CI'(aq) 6. Calculat... 2req (2) Write a balanced equation for the overall redox reaction. Use 7. Relation... 2req smallest possible integer coefficients. 8. Calculat... 2req + 9. Calculat... 2reqarrow_forward
- Give clear handwritten solution of the 1st and 2nd subparts...arrow_forwardEnter the following oxidation-reduction reaction into the answer blank. Be sure to use proper formatting and to include physical states. 2Ag" (ag) + Cu(s) –→ 2Ag(s) +Cu²* (ag) X| x. He -, fagl. 8.arrow_forward2. From the reagents below, construct balanced net ionic equations for: CF (aq), HCI (aq), NH4+ (aq), NH3 (aq), PbCl2 (s), Pb2+ (aq), Pb (s), Cr3+ (aq), Cr (s), CH:ОН (аq) 2a. A redox reaction. 2b. A precipitation reaction. 2c. An acid/base reaction.arrow_forward
- Consider the following unbalanced, net ionic, reduction-oxidation expression MnO4- (aq) + Cl2 (g) --> Mn2+ (aq) + ClO- (aq) A) Balance the Mn containing half-reaction in acidic solution. (You do not need to include the state symbols.) MnO4- --> Mn2+ B) Balance the Cl containing half-reaction in acidic solution. (You do not need to include the state symbols.) Cl2 --> ClO-arrow_forwardFor the following redox reactions, please balance them in basic solution and indicate the impactof pH on the reaction (e.g., lower pH favors, disfavors, has no effect on the reaction).arrow_forwardIn the reaction 2 Al + 5 H20 + OH + NO2 => NH3 + 2 Al(OH)4 The reducing agent is: O NH3 O OH OAI ) Al ONOarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY