
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Give detailed Solution with explanation needed
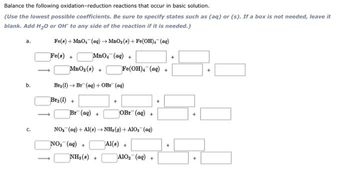
Transcribed Image Text:reactions that occur in basic solution.
Balance the following oxidation-reduction
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it
blank. Add H₂O or OH to any side of the reaction if it is needed.)
Fe(a) + MnO₂ (aq) →MnO₂ (s)+Fe(OH), (aq)
Fe(s) +
MnO₂ (aq) +
a.
b.
C.
MnO₂ (s) +
Bra (1) Br (aq) + OBr
Bra (1) +
Br (aq) +
NH₂(0)+
Fe(OH)4 (aq) +
(aq)
+
OBr (aq) +
NO₂ (aq) + Al()→ NH₂(g) + AlO₂ (aq)
NO₂ (aq) +
Al(s) +
A1O₂ (aq) +
+
+
+
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. Compounds that have an effective depigmenting effect are…A. ascorbic acidB. kojac acidC. azelaic acidD. all answers are correctarrow_forwardGive detailed Solution with explanation needed...don't give Handwritten answerarrow_forwardUsing the electron-pushing diagram shown, draw the product of the reaction, including nonbonding electrons and charges where appropriate. a H B H H Draw the product. Add charges and electrons where needed. Select Draw Rings More 3 с H B O Erase Q2 Qarrow_forward
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardGive detailed Solution with explanation needed with structures..don't give Handwritten answer..don't use Ai for answering this....arrow_forwardList the given compounds in order of increasing acidity (lowest first). And please explain the reason behind itarrow_forward
- Show work..give correct detailed Solution with explanation needed..don't give Handwritten answer..don't use Ai for answering thisarrow_forwardGive correct detailed Solution with explanation needed..don't give Handwritten answerarrow_forwardGive clear detailed Solution with explanation needed of all with structures..don't give Handwritten answer...arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY