
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
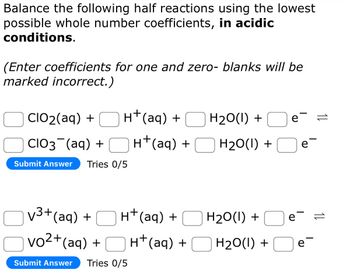
Transcribed Image Text:Balance the following half reactions using the lowest
possible whole number coefficients, in acidic
conditions.
(Enter coefficients for one and zero- blanks will be
marked incorrect.)
H+ (aq) +
CIO₂(aq) +
CIO3¯(aq) +
Submit Answer Tries 0/5
√³+ (aq) +
vo²+(aq) +
Submit Answer Tries 0/5
H+ (aq) +
H+ (aq) +
H+ (aq) +
H₂O(l) +
H₂O(l) +
H₂O(l) +
H₂O(l) +
e =
e
e=
e-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 12 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance the following half-reactions by adding the appropriate number of electrons (e-). Then, classify each reaction as an oxidation or reduction half-reaction. Note that for each of the four reactions, one of the gray boxes will be left blank and the other will be filled with electron(s). Use the symbol e- to represent an electron. Part 4 + 12(s) + 6H2O(1) > 2I0-3 + 12H+(aq) + --arrow_forwardUsing standard reduction potentials from the ALEKS Deta tab, calculate the standard reaction free energy AG for the following redox reaction. Round your answer to 3 significant digits. Cu (aq) + HNO₂ (aq) + H* (aq) → Cu² (aq) + NO(g) + H₂O (1) Xarrow_forward3:19 PM Thu May 4 * 8 J ↑ M ● ( 9 K 8 H x H₂O O O 9 L cmd = (aq) P H3O+ "' X 0 : ; { [ 300 option ? 1 50 69% Ï Submit 1 delete 1arrow_forward
- Write balanced half-reactions for the following redox reaction: 2+ 2+ 3+ MnO4(aq) +8 H* (aq) +5 Fe²+ (aq) Mn²+ (aq) + 4H₂O(1)+5 Fe³+ (aq)arrow_forwardFunctional Group Type of Compound Name Hydroxyl group Alcohol Ethan___________ Amino group Amine Ethan___________ Carboxyl group Carboxylic acid Ethan___________ _______ Carbonyl group Aldehyde Ethan___________arrow_forwardPlease answer letters D, E, F only in the attached picarrow_forward
- Examine the following half-reactions and select the strongest oxidizing agent among the substances. v Jersey Departm E° = 0.755 V [PtCl4]²¯(aq) + 2e¯ 2 Pt(s) + 4CI'(aq); RuO4(s) + 8H*(aq) + 8e¯ 2 Ru(s) + 4H2O(1); FeO4²(aq) + 8H*(aq) + 3e¯ 2 Fe3*(aq) + 4H2O(/); H4XEO6(aq) + 2H*(aq) + 2e¯ 2 XeO3(aq) + 3H2O(/); E° = 2.42 V E° = 1.038 V E° = 2.07 V RuO4(s) H4XEO6(aq) [PtCl4]² (aq) O HFEO4¯ (aq) CI(aq)arrow_forwardEnter electrons as e". Use smallest possible integer coefficients. If a box is not needed, leave it blank. For the following electron-transfer reaction: Br2(1) + Mn(s) → 2Br"(aq) + Mn²+(aq) The oxidation half-reaction is: + > The reduction half-reaction is: + +arrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S2O3² (aq) → S4O6²¯(aq) 1 + 4- 2 Reset 0₂ H OH- ų 3 ( ) 0²- 4 e S 5 6 05 ☐6 1L (s) H+ 2+ H₂O 8 18 4+ x H₂O 0 ☐ ☐o (1) (g) (aq) H3O+ Deletearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY