
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
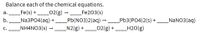
Transcribed Image Text:Balance each of the chemical equations.
Fe(s) + 02(g) → Fe203(s)
_Na3PO4(aq)
_NH4NO3(s) –
а.
b.
_NaNO3(aq)
Pb(NO3)2(aq) –
N2(g) +
Pb3(PO4)2(s) +
_H2O(g)
C.
--- ____02(g) +
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 1. Balance the following chemical equations. _Na;PO4 (aq) +_Al(NO3)3(aq) _NaNO3 (aq) + AIPO4 (s) a. b. _Pb(NO3)2 (aq) + KI (aq) _Pbl2 (s) + KNO; (aq) с. Fe (s) + CUSO4 (aq) Cu (s) + _Fe2(SO4)3 (aq) d. _KC103 (s) KCI (aq) + _O2 (g) е. _Fe,O3 (s) + _H,O (1) Fe(ОH); (s) f. _Са(ОН), (аq) + _HNO3 (aq) _Ca(NO3)2 (aq) + _H,O (1I) 2. Classify each of the following reactions as combination (C), decomposition (D), single replacement (SR), or double replacement (DR). H2 (g) + Cl2 (g) → 2 HCl (g) а. b. PBC12 (aq) + 2 KI (aq) Pbl2 (s) + 2 KCI (aq) с. 2 Na3PO4 (aq) + 3 CUSO4 (aq) Cu3(PO4)2 (s) + 3 Na,SO4 (aq) d. (NI CO3( 2 NH3 (g) + CO2 (g) + H2O (1) Cu (s) + 2 ZNNO3 (aq) Cu(NO3)2 (aq) + 2 Zn (s) e.arrow_forwardName: Balancing Chemical Equations Balance the following equations using whichever method you prefer: 1) K (s) + MgBr2 (aq) → KBr (aq) + Mg (s) 2) CHSOH (I) + CO2 (g) + H20 (g) 3) P (s) + (a) 30 P20s (s) 4) CO: (g) + H2O (1) > O2 (g) + CH12O6 (s)arrow_forwardAktiv Chemistry → C ock STARTING AMOUNT + esc X https://app.101edu.co Q X N T Aktiv Chemistry 2 W S #3 X 20 F3 H E 60.5 D X + Calculate the theoretical yield in grams All, from the complete reaction of 113 grams I, according to the following balanced chemical equation: ADD FACTOR $ 4 x( ) 0.445 mol All F4 R F LL do LO % 5 113 mol I₂ F5 V 2 Al(s) + 3 (s)- -> 153.88 T Question 8 of 17 6 1 G g/mol All, 253.80 Y B 2 7 g/mol I₂ H 2 All,(s) ANSWER 407.68 F7 D 3 8¹2 N CO 8 J DII FB RESET g All 2 0.297 9 M DD K F9 121 O 7 H F10 L P F11 LL 11 F12 U ☆ delearrow_forward
- 13. For each of the following balanced chemical equations, calcu- late how many grams of the product(s) would be produced by complete reaction of 0.125 mole of the first reactant. a. AGNO,(aq) + LIOH(aq) → AgOH(s) + LINO,(aq) b. Al;(SO)(aq) + 3CAC1;(aq)→2AICI,(aq) + 3CASO.) c. CaCO3(s) + 2HCI(aq)→CACI(aq) + CO,(g) + H;0(1) d. 2CH10(8) + 130;(g)→8CO;(g) + 10H,0(g) 14. For each of the following balanced chemical equations, calcu- late how many moles and how many grams of each product would be produced by the complete conversion of 0.50 mole of the reactant indicated in boldface. State clearly the mole ratio used for each conversion. a. NH3(g) + HCI(g)→ NH,Cl(s) b. CH,(g) + 4S(s) CS2(1) + 2H,S(g) c. PCI3(1) + 3H,O(1) → H,PO;(aq) + 3HCI(aq) d. NaOH(s) + CO:(g)NaHCO;(s) 15. For each of the following unbalanced equations, indicate how many moles of the second reactant would be required to react exactly with 0.275 mole of the first reactant. State clearly the mole ratio used for the…arrow_forwardBalance the following equationsarrow_forwardThe following precipitation reaction is perfomed using 3.785 g of FeCk and 2.258 g of NaOH. FeCk (aq) +3 NaOH (aq) Fe(OH): (s) 3 NaCl (ag) a. What is the theoretical yield of the produce (solid) in this reaction? b. If the reaction produces 1.825 g of the product, what is the percent yield? c. Write the full and net ionic equations for this reaction.arrow_forward
- Write the balanced chemical equation for the following chemical reactions. Include the correct formulas for all reactants and products and balance with coefficients. Identify the type of reaction in the right column. bbs yd anal Balanced Equation 11. Iron and oxygen react to form iron (III) oxide. OCH Fet 302 12. 2 Fe203 Ag No 3 t. MgCl2 → Mg (NO 3 ) ₂ + Ag Cl 100 H 2 + onjа ин erit anionels (a)tubara en ho (e)slümot dit gnishw bubong salt goinlinereb di bis 87 Holdsset to says arls noised to sayI temessige eldung A equations for the either the read odstrabi 18229090 Type of Reaction Silver nitrate and magnesium chloride react to form magnesium nitrate and silver chloride. Double displacement reaction 13. Aluminum reacts with copper (II) sulfate to form aluminum sulfate and copper solid. OCH H The Redox reaction 01019 ion 02 H OVBA noitsup3 beonsle8 upe ghiwollet od: 919lamo zinaibittooo ritiw noitsupe +Del Calld OSH TOVEN TO 350°F BAKING SMEROM BOX AND d only for orders at…arrow_forward2.80 grams of aluminum foil is mixed with 104.0 mL of 2.00 M CuSO4 solution. The reaction is 2Al(s) + 3CuSO4(aq) >> 3Cu(s) + Al2(SO4)3(aq) a. Determine the limiting reactant b. What mass of Al(s) and CuSO4 are consumed? c. What mass of Cu(s) is formed? If experimental yield of Cu(s) is 9.20 g, determine the percentage yield of Cu(s).arrow_forwardNa2CO3 + 2HCl --> CO2 + H2O + 2NaCl Mass of beaker + indicator = 76.730g Mass of beaker with indicator and sodium carbonate = 77.827g Net mass of sodium carbonate = 1.097 What is the Theoretical yield of sodium chloride?arrow_forward
- Write the balanced NET ionic equation for the reaction when aqueous calcium chloride and aqueous lead(II) nitrate are mixed in solution to form aqueous calcium nitrate and solid lead(II) chloride. 04- 3- D²- 134 口4 1. 6 7. 口。 国。 (s) () (aq) C CI Pb Ag H. Ca 1L 3.arrow_forwardVisited 2011 147*70 Use the References to access important values if needed for this question. of silver nitrate? According to the following reaction, how many grams of copper are required for the complete reaction of 30.8 grams 2AgNO3(aq) + Cu(s) → Cu(NO3)2 (aq) + 2Ag(s) Q Search Y H Submit Answer g copper & Retry Entire Group De a 18 00 fo K S B O hp 9 more group attempts remaining ho 6 ugd 11 111 (12 ? + ins prt sc delete 2 ✰ ✰ 0 Pre Previous Next> ^ G backspace 244 home lock end 7 5:34 PM 5/14/2023 1## Aarrow_forwardArsine, AsH3, was once a product of a test used in forensic analysis. Tissues from a possible poisoning victim were treated with zinc and sulfuric acid. If arsenic was present (usually as diarsenic trioxide, As203), arsine would form: As₂03 (s) + 6 Zn(s) + 6 H₂SO4(aq) ----> 2 AsH3(g) + 6 ZnSO4(aq) + 3 H₂O(1) a) In a lab exercise, a forensic technician reacts 19.8 g of AS₂O3 with 32.7 g of Zinc in the presence of excess sulfuric acid. What mass of AsH3 will be produced? b) Use the same chemical reaction, create a question which needs to be solved by percentage yield.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY