
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
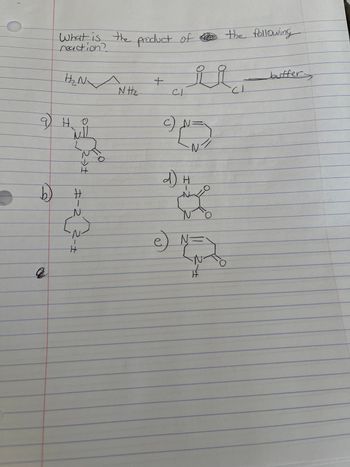
Transcribed Image Text:-
b)
Q
What is the product of the following
H₂N
но
MI!
H
キー
N
N Hz
+
CI
c)
معد
N/
a
e) N =
N
buffer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is one acidifying process that produces H+ ions?arrow_forwardA solution may contain any or all of the following cations: Ag+, Pb2+, Ba2+, Ni2+ in an UNKNOWN mixture. HCl is added to the solution and a precipitate forms. That precipitate is removed and NH3 (aq) is added to the supernatant until the solution is basic, no solid forms. After making the supernatant basic, K2CrO4 is then added and a precipitate forms. What cations (Ag+, Pb2+, Ba2+, Ni2+)are present in this unknown sample (This is chemistry and not a writing question)arrow_forwardConsider the following reactions: Reaction 1: HCIO, + KOH → KC1O, + H;O Reaction 2: HC;H;O2 + KOH → KC;H;O2+ H;O In both reactions, 15.00 mL of 0.8116 M acid is titrated with 0.4217 M KOH. A volume of 28.87 mL of KOH is required to reach the equivalence point in both reactions. a) What is the pH of the HC1O, solution before the titration has started? (No KOH has been added.) b) What is the initial pH of HC;H;O2 solution before the titration has started? K= 6.46 x 10-5 (Ok to use x is small.) c) What is the pH of Reaction 1 at the equivalence point? d) What is the pH of Reaction 2 at the equivalence point? e) What is the pH of Reaction 1 after 32.00 mL of KOH has been added? f) What is the pH of Reaction 2 after 18.00 mL of KOH has been added?arrow_forward
- What is the [H3O*] in a 175 mL sample of 0.0629 M KOH(aq)? 6.08 x 10-13 mol L-1 8.17 x 10-14 mol L-1 2.78 x 10-14 mol L-1 1.59 10-13 mol L-1 9.08 x 10-13 mol L-1arrow_forwardPlease write a balanced equation and indicate the state. HCl(aq) + H2O (l)arrow_forwardA 50.0 mL solution of Ca(OH)₂ with an unknown concentration was titrated with 0.340 M HNO₃. To reach the endpoint, a total of 28.4 mL of HNO₃ was required. Given that 0.00966 mol of HNO₃ are used in the titration, how many moles of Ca(OH)₂ had to be present in the initial reaction?arrow_forward
- show the balanced general equation, used indicator, the type of reaction, and unknown concentration: Titration of 10 mL of 0.4N HCl solution with 20 mL NaOH. What is the concentration of NaOH? Titration of 20 mL H2O2 solution with 10 mL 0.1 M KMnO4. What is the concentration of the solution of H2O2 in percentage (mass/volume)?arrow_forwardWhat are the correct reaction conditions for the following transformation? CI HO Na Na ? For + HCIarrow_forwardWhich of the following aqueous solutions are good buffer systems? O0.13 M hypochlorous acid + 0.12 M sodium hypochlorite O 0.10 M potassium fluoride + 0.30 M hydrofluoric acid 0.27 M hydrobromic acid + 0.23 M potassium bromide 0.39 M acetic acid + 0.28 M potassium acetate O 0.34 M ammonium nitrate + 0.38 M ammoniaarrow_forward
- 5 b) Benzoic acid, HC7H5O2, is a weak monoprotic acid. A stock HC,H5O2 solution with unknown concentration was prepared. A 25.00 mL sample of the stock HCH5O2 solution was titrated with 0.1234 M sodium hydroxide to the equivalence point, using phenolphthalein as an indicator. The titration took 20.26 mL of titrant (sodium hydroxide). (b) Write a balanced complete ionic equation for the chemical reaction that occurs in this titration.arrow_forwardWhich of the following aqueous solutions are good buffer systems? O 0.34 M ammonium nitrate + 0.31 M ammonia O 0.20 M sodium hydroxide + 0.23 M sodium chloride O 0.37 M hydrofluoric acid + 0.25 M sodium fluoride O 0.17 M nitrous acid + 0.11 M sodium nitrite O 0.21 M perchloric acid + 0.17 M sodium perchloratearrow_forwardWhich of the following aqueous solutions are good buffer systems? O 0.36 M hydrofluoric acid + 0.23 M potassium fluoride O 0.27 M hydrochloric acid + 0.22 M sodium chloride O 0.15 M potassium hydroxide + 0.24 M potassium chloride O 0.15 M hydrocyanic acid + 0.15 M sodium cyanide O 0.27 M ammonium bromide + 0.33 M ammoniaarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY