
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Topic Video
Question
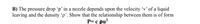
Transcribed Image Text:B) The pressure drop 'p’ in a nozzle depends upon the velocity 'v’ of a liquid
leaving and the density 'p'. Show that the relationship between them is of form
P= c pu?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A pipe transports gas up a hill. At the bottom of the hill, the mean velocity in the pipe is 10m/s and the pressure is 200Pa. The pipe is then raised 10m to the top of the hill, where the pressure is now measured to be 50Pa. Find the velocity at the top of the hill. The density of the gas is 1.1kg/m^3, and the acceleration due to gravity is 9.81m/s^2arrow_forwardHelium gas is sold in steel tanks that will rupture if subjected to tensile stress greater than its yield strength of 5 × 108 N/m2. If the helium is used to inflate a balloon, could the balloon lift the spherical tank the helium came in? Justify your answer. Suggestion: You may consider a spherical steel shell of radius r and thickness t having the density of iron and on the verge of breaking apart into two hemispheres because it contains helium at high pressure.arrow_forwardS:44)arrow_forward
- A sphygmomanometer is a device used to measure blood pressure, typically consisting of an inflatable cuff and a manometer used to measure air pressure in the cuff. In a mercury sphygmomanometer, blood pressure is related to the difference in helghts between two columns of mercury. The mercury sphygmomanometer shown in the figure below contains air at the cuff pressure P. Po The difference in mercury heights between the left tube and the right tube is h= 109 mmtg - 0.109 m, a normal systolic reading. What is the gauge systolic blood pressure Poauge in pascals? The density of mercury is p 13.6 x 10 kg/m and the ambient pressure is Po1.01 x 10 Pa. HINT Paarrow_forwardIn scuba diving, a regulator is used so that the pressure of the air the diver breathes is close to that of the ambient water. A reckless swimmer decides to use a hose sticking out of the surface to breathe underwater while diving in a lake. When the air pressure in the lungs is at a pressure of around 0.150 atmospheres below the ambient pressure, lung injury may occur. Find the depth at which the swimmer would experience such a pressure differential.arrow_forward19. The radius of the bronchial tube is decreased by a factor of 4. The velocity of gas through it initially is 1 m/s. What is the velocity in the section of bronchial tube with decreased radius in m/s?arrow_forward
- An aquatic organism needs to be neutrally buoyant to stay at a constant depth. Fish accomplish this with an internal swim bladder they can fill with air that they take in from the water through their gills. One complication is that the pressure in the swim bladder matches that of the surrounding water, but the water pressure changes with depth. Because the volume of a gas is inversely proportional to pressure (as you may already know if you have studied the ideal-gas law), the volume of air in a fish's swim bladder decreases with depth unless the fish actively adds more air.arrow_forwardA vertical, frictionless piston-cylinder device contains a gas at 600 kPa absolute. The atmospheric pressure outside is 100 kPa absolute. The piston has a diameter of 50 mm. a) Determine the mass of the piston. b) If the mass of the piston is now halved ---- what would be the gauge pressure of the gas?arrow_forwardA simple thermometer is formed by taking a large spherical container of volume 1.13m³ and filling it with an ideal gas. Then, a U-shaped tube is attached that is very narrow so that very little fluid/gas can fit inside the tube. The tube has a small quantity of a fluid of density 1200kg/m³ placed in it. In equilibrium at room temperature - 21.9°C - the height of the fluid on both sides of the U-shaped tube are level. This is illustrated in the image below. What is the temperature of the gas in the spherical container when the left side of the fluid is 13.25cm higher than the right side of the fluid in the U-shaped tube? Atmospheric pressure is 101000Pa. °Carrow_forward
- An aquatic organism needs to be neutrally buoyant to stay at a constant depth. Fish accomplish this with an internal swim bladder they can fill with air that they take in from the water through their gills. One complication is that the pressure in the swim bladder matches that of the surrounding water, but the water pressure changes with depth. Because the volume of a gas is inversely proportional to pressure (as you may already know if you have studied the ideal-gas law), the volume of air in a fish's swim bladder decreases with depth unless the fish actively adds more air. ▼ Part A Consider a 3.9 kg freshwater fish whose tissues have an average density of 1050 kg/m³. To what volume in mL must the swim bladder be inflated for the fish to be neutrally buoyant at the surface? Express your answer in milliliters to two significant figures. AV = Submit VD] ΑΣΦ Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining ? mLarrow_forwardYou have a tube where water enters at a velocity of 10.0 m/s, ascends 5.0 m, and then exits. The pressure pushes the water in at 250 kPa, and is measured come out at 150 kPa. The outcoming water is, faster than the incoming water. slower than the incoming water. the same speed as the incoming water.arrow_forwardConsider a large 10.0 kg striped bass swimming in fresh water. When neutrally buoyant, 10% of the total volume of the fish is taken up by air im the swim bladder. Assume a constant bodyy temperature of 20 degrees C. and neglect the mass of the air in your calculations. a) What is the volume of the swim bladder, in m^3, when the fish is neutrally bouyant? B) What is the water pressure at a depth of 25m? C) How many moles of air ar in the swim bladder when the fish is neutrally bouyant at a depth of 25m? D) What will the volume of the swim bladder be if the fish ascends to a depth of 15m without changing the quantity of gas?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON