
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
B. Draw the structure of the synthesized product (do not include side or by products) from the experiments performed. How can IR spectroscopy be used to monitor the progress of each of the following reactions (if reaction went to completion and/or check the purity of the final product)? What absorptions would be observed in the IR spectrum? And if IR spectroscopy could not have been used, briefly explain why.
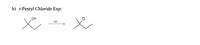
Transcribed Image Text:b) t-Pentyl Chloride Exp:
OH
HCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. In both the starting material and product the chemical shift of the hydroxyl proton is approximately 13 ppm. Table 22.2 in your “Techniques in Organic Chemistry" textbook gives 4.0 - 8.0 ppm as the typical range for phenolic protons. Explain this apparent discrepancy. Hint: Think about the solvent the NMR spectrum was run in.….arrow_forwardLAB Q4. Draw the structure of the synthesized product (do not include side or by products). How can IR spectroscopy be used to monitor the progress of each of the following reactions (if reaction went to completion and/or check the purity of the final product)? What absorptions would be observed in the IR spectrum? And if IR spectroscopy could not have been used, briefly explain why.arrow_forward3. Briefly explain how would you distinguish between the IR spectra of the following two compounds using IR spectroscopy? Be specific and explain why there is a difference.arrow_forward
- Consider the product below a) Would this product show a signal in a UV-Vis spectrum? Why? b) Would this molecule have a non-zero alpha-value in polarimetry? Why? c) What stretches would be expected in an IR spectrum of this molecule? d) Predict the 1H NMR spectrum for this molecule. For H1 to H6 e) Predict the 13C NMR spectrum and DEPT spectra for Carbon A- Carbon G. Use (+) for positive signals, (-) for negative signals, and (X) for no signal in the DEPT NMR spectra. f) If a base such as NaOH was used, it would be harder to determine the major product before running the reaction. Once the reaction was run, how would you determine the product favored using spectroscopy?arrow_forwardA student attempted to create the following conjugated compound via a double elimination. The mass spectrum showed the correct compound had been formed. And yet, the IR spectrum should definitive evidence that the wrong compound was formed. Answer using the bold letters. What do you think was formed ? Which functional group? A alcohols B alkyne C ketone D another functional group What peak was lacking from the IR spectrum that should have been there E 1500-1800cm1 F 2000-2500cm-1 G 3000-3500cm1 What peak was present in the IR spectrum that confirmed the wrong product H 1500-1800cm1 I 2000-2500cm1 J 3000-3500cm1 Br 2eq NaOHarrow_forwardMatch the four structures with the four IR spectra (by putting a proper letter in the box to the right of each spectrum). Use specific IR absorptions for various functional groups to identify the structures. Circle the important peaks in each spectrum that helped you identify the functional group(s) and label the functional group beside it.arrow_forward
- c. Can the carboxylic acid derivative shown as a reactant in part a be used make an anhydride? d. Can product A be used to make a carboxylic acid? e. What type of carboxylic acid derivative iss product A? f. Using IR spectroscopy, how would you determine the reaction in part a produced A? (Compare the expected IR spectra for the reactant and product structures to provide the best answer. Be specific.)arrow_forward24. How can IR spectroscopy help to monitor the following reaction? OH e PCC a. What structural features distinguish the reactant from the product? b. What structural features would NOT help in distinguishing the two structures? C. What would you look for in the IR data of both structures? d. How would you know when the reaction was complete?arrow_forward2. READ THE DIRECTIONS TO EACH QUESTION CAREFULLY - not following the directions means you get the question wrong! 3. FORMAT YOUR ANSWERS AS DIRECTED-formatting your answers incorrect means you get the question wrong! 4. Below are the IR regions and NMR chemical shifts table for use with the spectroscopy problems: ¹H NMR 12 11 10 R C=O ... OH R 'H 9 8 7 H ppm 6 OH C=C-H 5 NH H-C-X H-C-O -OH -NH X = F, Cl, Br (i.e. electronegative atom) 3 2 H-C-N H-C-S -CH3 -CH₂- C=C-H H-C-C=O -CH H-C-C=C HC-0 0 4000 DIO small range range of values broad peak =C-H N-H 3250- 3300 broad with spikes -3300 3500 -O-H broad-3300 -3400 O-H -CN usually strong N-H CO =C H H 2730- 2820 3000- 2 peaks 3100 1 H -C-H 2850- 2960 O 0 -C-O-H broad-3000 3000 -CEN 2200 -CICH ← 2200 2500 wavenumber, cm-1 2000 C H.C. N н 1680 0.00 0.00 0.00 H 1600- 1660 O C. OR 2 :ں 1600 1500 1000arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY