
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
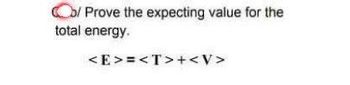
Transcribed Image Text:Co/Prove the expecting value for the
total energy.
<E>=<T>+<V>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Homework 6 Problem 4: A mass m of water is heated reversibly from temperature T1 to T2 at a constant pressure of P. In this problem, we are going to determine an expression for the change in entropy, ΔS.Assume we can heat the given water infinitesimally slowly so that the process is reversible. Therefore, heat in any infinitesimal step is given by the following: dQ = mc dT, where c is the specific heat and is constant. Part (a) Write an expression for the change in entropy ΔS for the system. Part (b) Calculate the change in entropy in cal/K for a sample of water with mass m = 1.6 kg and changing temperature from T1 = 25.6°C to T2 = (25.6+10)°C. The specific heat c of water is 1,000 cal/kg/K. ΔS = ______arrow_forwardUsing Kirchoff's Rules determine power dissipated by R4 if: R1: 8ohm, R2: 4ohm, R3: 14ohm, R4: 10ohm, R5: 12ohm, V1: 11V, V2: 14Varrow_forwardIf 370 J of work is performed with 45% efficiency, what is the amount of energy that goes into heating, parasitic losses, etc.? Give your answer in units of joules, to three significant figures.arrow_forward
- 4) Physical systems work in a “one-way street” – the amount of information we have about the state (energy state, position, velocity, etc.) of the molecules, atoms, and particles involved in general, decreases over time. A measure of the decrease of this type of information over time, or the increase in disorder, is called: energy force the adiabatic constant entropy workarrow_forwardS(B, T) 1 T₁ T Figure A1 Figure A1 shows the entropy S of a paramagnetic solid as a function of its temperature T and imposed magnetic field B. Adiabatic cooling consists in bringing the system from state 1 to 3 by making it undergo an isothermal change of magnetic field from 1 to 2 followed by an isentropic adiabatic process from 2 to 3. The entropy of the solid can be approximated by the equation S(B,T) = So — K B² T2' - where K is a positive constant and Så is some reference entropy value. (a) Find the expression of the heat Q₁2 received by the system during the isothermal process from state 1 to 2. Discuss the meaning of its sign. (b) Express the final temperature T3 as a function of B₂ and T₁. 3 T3 B₁ < B₂ B₂arrow_forwardFor each of the following situations: a. Choose an initial and final state (states A and B) that will be appropriate (interesting) for analysis. b. If there is not already a physical diagram of the situation and there should be one for proper analysis, include one. c. Construct a system schema of the situation. Do not forget to include the system boundary for the system specified by the problem. Choose your system wisely. d. Construct an energy bar chart and system flow diagram (LOL plots) for each problem. e. You are allowed to add other energy storage modes. You do not have to use the energy storage modes that are printed in each LOL plot. f. Find the unknown value.arrow_forward
- A power plant has been proposed that would make use of the temperature gradient in the ocean. They system is to operate between 24.0°C (surface water temperature) and 4.95°C (water temperature at a depth of about 1 km). a. What is the maximum efficiency of such a system? Describe the process that would be used to get this maximum efficiency. b. If the useful power output of the plant is 80.0 MW, how much energy is absorbed per hour?arrow_forwardI Review I Constants I Periodic Table A 68 kg hiker walks at 5.0 km/h up a 6 % slope. The indicated incline is the ratio of the vertical distance and the horizontal distance expressed as percentage. Part A For the steps and strategies involved in solving a similar problem, you may view a Video Tutor Solution. What is the necessary metabolic power? Hint: You can model her power needs as the sum of the 380 W power to walk on level ground plus the power needed to raise her body by the appropriate amount. Assume that the efficiency of the body in using energy is 25%. Express your answer with the appropriate units. HẢ ? P = Value Units Submit Request Answerarrow_forwardCritically ill patients in a cardiovascular intensive care unit must be intensely monitored for any number of conditions. One of these conditions is hypoperfusion, which is an overall decrease in blood flow throughout the body. Low stroke volume is one indicator of hypoperfusion. The pressure-volume (PV) graph below shows three possible loops for the heart. The control loop shows a healthy heart while the other two loops show reduced or increased preload (assuming that afterload and contractility remain fixed). 200 LV Pressure (mmHg) 100 Preload Control ↑ Preload 100 LV Volume (ml) (a) What is the stroke volume for the control patient? RK '15 200 (b) A healthy heart rate (HR) is measured as 80 beats per minute. What is the control patient's cardiac output CO? Control patient's CO: mL minarrow_forward
- Helparrow_forward4. Suppose that a nuclear power plant has an efficiency of about 0.34, and generates 1000 MW of power. It is located on the banks of a major river that is 67 m wide near the plant, approximately 3 m deep, and flows at a rate of 0.5 m/s. Suppose that the plant re-routes all of this water into the plant and dumps its waste heat evenly throughout the water, then returns the warmer water to the river. How much warmer is the river downstream of the plant compared to upstream? Water's specific heat is approximately 4184 J/kg/K, and its density is 1000 kg/m³. [Answer: The water is warmer by 4.62 K. This is a lot warmer!]arrow_forward(c) We are asked to isolate t [work shown below]. Vors Ve cos Q Voy- : V, sin e Ranger X= Vore t x Vz Cos E . € cos Afso AY= Voy't hes Va sin @it . he= Vz sin Ô .t-4,9€² 4.9¢² -Vz sin@etphz20 9.01 Then to plug it into y (1) = yo + Vo,yt +;a,t“. I am hoping for help on the second step.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON