
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
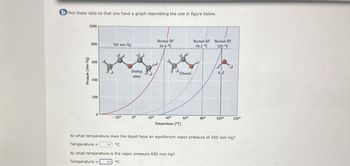
Transcribed Image Text:b Plot these data so that you have a graph resembling the one in figure below.
Pressure (mm Hg)
1000
800
760 mm Hg
Normal BP
34.6 °C
Normal BP
78.3 °C
Normal BP
100 °C
600
Diethyl
Ethanol
H₂O
ether
400
200
0
-20°
0°
20°
40°
60°
80°
100°
120°
Temperature (°C)
At what temperature does the liquid have an equilibrium vapor pressure of 250 mm Hg?
Temperature =
°C
At what temperature is the vapor pressure 650 mm Hg?
Temperature=
°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- ✓ 3 The enthalpy of vaporization of Substance X is 30.0 Round your answer to 2 significant digits. kJ and its normal boiling point is 99. °C. Calculate the vapor pressure of X at -87. °C. molarrow_forwardFind the enthalpy of vaporization, ΔHvap.arrow_forwardUse the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.3 atm. کارا pressure (atm) solid ]°C 200 liquid gas temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. X 400arrow_forward
- The pressure above a pure sample of solid Substance X at -180. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to find your answer. pressure (atm) 0.4- 0.2- solid liquid 0. 200 temperature (K) gas 400 Note: your answer must be within 0.025 atm of the exact answer to be graded correct. atm хarrow_forwardA chemist determined vapor pressures of an unknown substance at different temperatures. The data was plotted as ln(P) vs 1/T (temperature in Kelvin). What is △H∘vap of this substance in kJ/mol?arrow_forwardkJ The enthalpy of vaporization of Substance X is 10.0 and its normal boiling point is 110. °C. Calculate the vapor pressure of X at 33. °C. mol Round your answer to 2 significant digits. atm х10arrow_forward
- This graph shows how the vapor pressure of three liquids varies with temperature: 900 800 700 600 500 400 300 200 octane acetylacetone orthoxylene 100. 100 110 120 130 140 temperature, °C Use the graph to answer the following questions: Which liquid is the most volatile? most volatile: choose one Which is the least volatile? least volatile: choose one O°c octane: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. acetylacetone: orthoxylene: Suppose a beaker of acetylacetone is put inside a sealed tank containing acetylacetone gas at 119. degree C and 206. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? more less the same vapor pressure, torrarrow_forwardConsider the phase diagram shown. What is the normal sublimation point at 1 atm? Pressure (not to scale) 72.9 atm 5.1 atm 1 atm O 0°C O 100°C -56.7 °C 31°C -78.5 °C SOLID LIQUID GAS -78.5 °C -56.7 °C Temperature (not to scale) 31 °Carrow_forwardThis question is not a grade question, its just a pratice problem.arrow_forward
- Based on the graph which describes two liquids, A and B, select ALL of the FALSE statements. XA OA The substance with the higher pure vapor pressure (P") is B OB. The substance with the higher pure vapor pressure (P°) is A OC. For any mixture of A and B (any ratio of A and B), the mole fraction of B in the vapor phase will be larger than the mole fraction of B in the liquid phase. OD. An equimolar mixture of liquids A and B are placed in a closed container (25 °C). At equilibrium, the vapor above the mixture will contain more moles of B than A OE Substance B has greater intermolecular forces than A MacBook Pro esc %23 & 1 2 3 4 5 6 7 8 9. delete Q E R T. Y tab A D F G J K os lock > V B N M control option command command option vapor pressurearrow_forwardThe following compounds are liquid at -10 °C: diethyl ether, methanol, and ethylene glycol. Arrange the three compounds in order of increasing vapor pressure at -10 °C. Choose between 1 - lowest vapor pressure, 2, or 3 - highest vapor pressure. Note: Reference the Phase change properties of pure substances table for additional information. Ethylene glycol (Choose one) Methanol (Choose one) Diethyl ether (Choose one) Garrow_forwardIn addition to filling in the blanks below, show all of your work for this problem on paper for later upload. The heat of vaporization of isopropanol is 44.0 kJ/mol. The vapor pressure of isopropanol at 400 torr is 67.8 °C. Calculate the normal boiling point of isopropanol. Enter your value in the first box and an appropriate unit of measure in the second box.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY