
Structural Analysis
6th Edition
ISBN: 9781337630931
Author: KASSIMALI, Aslam.
Publisher: Cengage,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I need the answer as soon as possible
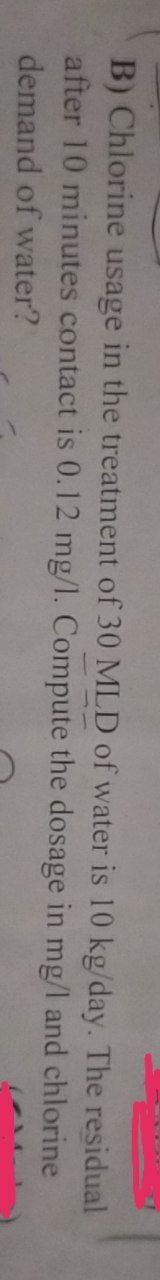
Transcribed Image Text:B) Chlorine usage in the treatment of 30 MLD of water is 10 kg/day. The residual
after 10 minutes contact is 0.12 mg/l. Compute the dosage in mg/l and chlorine
demand of water?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, civil-engineering and related others by exploring similar questions and additional content below.Similar questions
- Q4-B/ (6.0 Marks) In titration for alkalinity (5.0 ml) of 0.02N Sulfuric acid is used to reach the phenolphthalein end point and an additional (8.0 ml) to reach the mixed bromocresol green-methyl red end point for a(100 ml) water sample .Calculate the alkalinity species and values?arrow_forwardDetermine the preak point dosage of chlorine given the data below which was obtained from chlorination experiment. 5 6 7 Dosage(mg/L) Required Cl₂Cmg/L 0.2 0.45 1.05 2.75 4.5 5.15 6.03 1 2 3 4arrow_forward2.3 You have been asked to design a circular de-chlorination basin for 2 MGD treated wastewater. If use Na2S2O3 and the depth of the tank is to be 4.5 ft, what would you suggest the diameter of this tank? a) 50 ft c) not enough information 2.4 Alum required for a 1.5 mg/L dose and for a plant treat 10 MGD of drinking water is: a) 68.3 Ib/day b) 42 ft b) 125.11b/day d) no need for tank c) 62.6 Ib/day = d) 72.8 Ib/dayarrow_forward
- Liquid alum is used as a coagulant and specific gravity of alum is 1.33. One gallon of alum weights 6.05 kg and contains 3.22 kg of dry alum. Determine;(a)The alumconcentration (b)What is the volume of liquid alum required in order to prepare a solution containing 20,000 mg/l of alum concentration with final volume of 100 mL? (c)The dosage concentration of 1 mL of stock solution in a 2000 mL Gator jarsample.arrow_forwardIn standardization, 10.00 mL of Naz C2 04 solution required 39.17 mL of KMN04. Find the molarity of KMNO,. The unknown required 14.44 mL of MnO,. Find Ca in the urine. (Answer: 1.086 x 10 M, 7.840 x 10 M)arrow_forwardPlease include Equations used. This is for water and wastewater engineeringarrow_forward
- 2. You are asked to design a disinfection system with a capacity of 50,000 L/hr to achieve 3 log removal of C. parvum from a water source with a minimum annual temperature of 10 °C. Assume that you will use free chlorine at pH=6 and that you are designing to achieve a chlorine residual of 0.3 mg/L. Specify the total volume required (in m³) for the chlorine contactor and its dimensions (LxWxH in m)arrow_forwardProblem: You are designing a 20 mgd wastewater treatment plant. To characterize the plant's influent solids, the lab used 90 ml samples of the ty[ical wastewater and the following equipment: Solids: Lab Equipment Evaporation Dish Gooch crucible 2.0-micron paper filter 2.0-micron Whatman GF/C filter Lab Measurement Dish + Residue after evaporation 4. Weight 54.2830g 54.2170g 1.5240g 1.6948g 1. The lab placed a sample in an evaporation dish and evaporated it at 105°C finding that the dish weighted 54.3490 g after the dish cooled. What is the concentration (mg/L) of the Total Solids (TS) in the influent?(Ans: TS = 733.3 mg/L) 54.349g 2. If you anticipate a Total Suspended Solids mass loading of 48,400 lb/day at this flow rate, what is the concentration of TSS you anticipate in the influent? What does the analytical scale read? 3. The lab then filtered a sample through a 2-micron filter and evaporated the filter at 105°C, what was the mass of the residue found on the filter paper after it…arrow_forwardplease anser 7 and 8arrow_forward
- How many gallons of 12-percent sodium hypochlorite solution are needed to disinfect a reservoir 20 feet in diameter and 6 feet deep? An initial chlorine dose of 80 milligrams per liter (mg/L) is expected to maintain a chlorine residual of over 50 mg during the 24-hour disinfection period.arrow_forwardA wastowater sample of 100 ml. was filtered through the filter paper and the filtered wator was collected in a beaker. Based on the information provided in Table 1, calculato total solids, suspended solids, dissolved solids in wastowater (mg/1.). Table 1: Description Weight of filter paper (gm) Weight of tilter paper +solids (gm) after oven dry at 105 'C Weight of filter paper +solids (gm) after oven dry at 105 C Weight of beaker (gm) Weight of beaker + water after oven-dried at 105 "C (qm) Weight 0.05 0.1 7.76 50 51.5 O Suspended solids - 76.6 mg/; dissolved solids 15 mg/ and Total solids 91.6 mg/ Suspended solids 86.6 mg/L; dissolved solids - 15 mg/L and Total solids - 101.6 mg/ Suspended solids - 76.6 mg/L; dissolved solids = 10 mg/L and Total solids - 86.6 mg/L Suspended solids 70.6 mg/L; dissolved solids = 25 mg/L and Total solids = 95.6 mgt.arrow_forwardProblem 8 Given L0,mix=12 mg/L, DO mix=DOsat=11.33 mg/L, kd=0.3 d-1, and kr =0.4 d-1, find the following: (a) Initial oxygen deficit (Do) (b) Critical time on the oxygen sag curve (tc) (c) Critical deficit (Dc)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Structural Analysis (10th Edition)Civil EngineeringISBN:9780134610672Author:Russell C. HibbelerPublisher:PEARSON
Structural Analysis (10th Edition)Civil EngineeringISBN:9780134610672Author:Russell C. HibbelerPublisher:PEARSON Principles of Foundation Engineering (MindTap Cou...Civil EngineeringISBN:9781337705028Author:Braja M. Das, Nagaratnam SivakuganPublisher:Cengage Learning
Principles of Foundation Engineering (MindTap Cou...Civil EngineeringISBN:9781337705028Author:Braja M. Das, Nagaratnam SivakuganPublisher:Cengage Learning Fundamentals of Structural AnalysisCivil EngineeringISBN:9780073398006Author:Kenneth M. Leet Emeritus, Chia-Ming Uang, Joel LanningPublisher:McGraw-Hill Education
Fundamentals of Structural AnalysisCivil EngineeringISBN:9780073398006Author:Kenneth M. Leet Emeritus, Chia-Ming Uang, Joel LanningPublisher:McGraw-Hill Education
 Traffic and Highway EngineeringCivil EngineeringISBN:9781305156241Author:Garber, Nicholas J.Publisher:Cengage Learning
Traffic and Highway EngineeringCivil EngineeringISBN:9781305156241Author:Garber, Nicholas J.Publisher:Cengage Learning


Structural Analysis (10th Edition)
Civil Engineering
ISBN:9780134610672
Author:Russell C. Hibbeler
Publisher:PEARSON

Principles of Foundation Engineering (MindTap Cou...
Civil Engineering
ISBN:9781337705028
Author:Braja M. Das, Nagaratnam Sivakugan
Publisher:Cengage Learning

Fundamentals of Structural Analysis
Civil Engineering
ISBN:9780073398006
Author:Kenneth M. Leet Emeritus, Chia-Ming Uang, Joel Lanning
Publisher:McGraw-Hill Education


Traffic and Highway Engineering
Civil Engineering
ISBN:9781305156241
Author:Garber, Nicholas J.
Publisher:Cengage Learning