
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
please write neatly and explain thoroughly
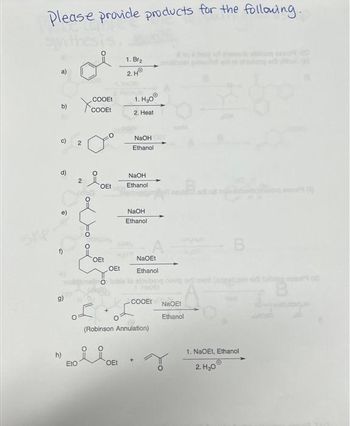
Transcribed Image Text:Please provide products for the following.
a)
b)
c)
d)
cy
2
COOEt
COOEt
EtO
OEt
OEt
OEt
+
1. Br₂
2.HⓇ
OEt
1. H₂0
2. Heat
NaOH
Ethanol
NaOH
Ethanol
NaOH
Ethanol
NaOEt
Ethanol
(Robinson Annulation)
ii
COOEt
pre
NeoEt
Ethanol
| bijvig brit mont (ajipatzman
r
g
B
1. NaOEt, Ethanol
2.H₂0Ⓒ
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give detailed Solution with explanation needed..don't give Handwritten answer..don't use Ai for answering thisarrow_forwardCrystals have precipitated upon cooling a homogeneous solution of a compound. The crystals were then separated by vacuum filtration. Which of the following terms is the best term to correctly to describe the liquid after filtration? Supernatant Solvent Solution Soluble impurities Filtratearrow_forward26. Give an example of when you might use decanting rather than filtration. Decanting is preferred for.... Write your answer here for example in Write your answer here. 27. Give an example of when you would filtration rather than decanting, Filtration is preferred for Write your answer here for example in write your answer here.arrow_forward
- Referring to your best straight line graph, what are the units of the slope?What physical property of steel is the slope of this line related to? Use the graphed data and your best trendline equation to predict the diameters of the three unknown ball bearings.Show your calculations below and report your three results in the data table above with 2 decimal place accuracy. ] 7. How could we improve the accuracy of the largest unknown ball bearing's diameter prediction? can you help me answer all this questions please, ai really appreciated it.arrow_forwardFor the Shaefer-Fulton endospore stain method, the working solution of safranin is made by combining 10 mL of stock solution with 90 mL of distilled water. If you need to produce 1,046 mL of working solution, how many grams of safranin O will be required to make the smallest batch of stock solution possible? • Report your answer in grams using standard decimal notation rounded to two decimal places. • Include trailing zeros. • Do not include the units in your answer 。 If your answer is 100.105 g it would be reported as 100.11 。 If your answers was 100.203 g it would be reported as 100.20arrow_forwardDuring the production of pure iron, limestone and coke are used. What are their purposes? (Inorganic Chemistry)arrow_forward
- What characteristics of a fired bullet are individualizing? The individualizing characteristics of a fired bullet are : caliber, rifling pattern, direction of twist of the lands and grooves, number of lands and grooves. IS MY ANSWER CORRECT?arrow_forwardP2O5 is called ________. N2O4 is called ________arrow_forwardPlease don't provide handwritten solution .....arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY