
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Write the mechanism for this
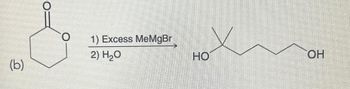
Transcribed Image Text:(b)
O
1) Excess MeMgBr|
2) H2O
HO
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- draw out the reaction as well as the mechanism for the reaction of methyl iodide and para-ethylphenol. Be sure to label the final productarrow_forwardDraw the mechanism and product for each of the reactions in Problem 18.45 when they are catalyzed by (a) base and (b) acidarrow_forwardShow the mechanism of the reaction of drawing 1 mole of water from the 2,2,5-trimethyl-3-hexanol compound, indicating the reaction conditions, step by step. Indicate the main product and by-product. b) Does the main product show the geometric isomer? If it does not show the isomers, please indicate why it does not. c) Write the products formed when the. main product ozonlamp is reduced.arrow_forward
- Please help provide an arrow pushing mechanism. The first step is to perform an electron transfer to form superoxide, but I am confused on where to go from there. isouramil e H₂O₂arrow_forwardWhat is the complete mechanism for this F!she rxn?arrow_forward1. what would be the mechanism? 2. what would be the product?arrow_forward
- i.) Ester hydrolysis can occur in acidic or basic conditions. Predict the product AND show the mechanism for the following addition/elimination reactions. Note that the first reaction is acid catalyzed and the second reaction is based catalyzed a.) " b.) مل NaOH H3O+ c.) Explain why an acid chloride reacts in neutral conditions to form the same product as 1 and 2 above. Show the mechanism for this hydrolysis reaction. WHY IS THIS REACTION NOT AN EQUILIBRIUM REACTION? CI H₂Oarrow_forwardShow the mechanism for the following reactionarrow_forwardCUTICLE OIL O Microsoft W final E.A 1. G fahrenheit x 9 Learning M X O Schoology x O ch 9 key A mukilteo.schoology.com/common-assessment-delivery/start/4592487484?action on.. Bb Molar Mie O Periodic x 3-6, 6-5, & 6-6 Test 6 of 10 © 29 POSSIBLE POINTS: 2 What is the mass of a 5.521 mole sample of MgCl2? O 17.26 g O 100.7 g O 525.7 g O 256.1 g 4. 6 8 9 10 Lenovo DI 23 $ 7 4 t r e ーの づarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY