
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Draw the nucleophile and electrophile precursor for each structure.
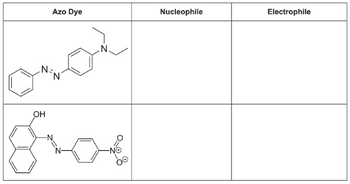
Transcribed Image Text:Azo Dye
N₂
N
OH
giat
Nucleophile
Electrophile
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given the general reaction coordinate diagram for an El reaction, what does the 1st transition state refer to? TS E TS, SM Reaction Coordinate An activated complex between the leaving group and carbocationic species. An activated complex between the nucleophile and carbocationic species. None of these O An activated complex between the base and carbocationic species.arrow_forwardShow the rate determining step for each an E1 and an E2 reaction including intermidiates / transition sites.arrow_forwardDraw ths missing curved arrow notation for the E2 reaction.arrow_forward
- e 2. Draw the structures and explain why CH3CH₂O and CH3CO₂ are good nucleophiles but CH3SO3, water, and alcohols (R-OH) are poor nucleophiles. Propose a 'cutoff for the amount of negative charge needed to be a good nucleophile. CH3CH₂O CH3CO₂ CH3SO3 H₂O CH3OHarrow_forwardPlease draw out the whole mechanism for the following reaction.arrow_forwardComplete the mechanism for the resonance stabilization. Draw the curved arrows, and complete the structure of the second resonance form.arrow_forward
- Determine which would be more reactive with a nucleophilearrow_forwardCreate a synthesis starting with the right side as the reagent and the left side as the productarrow_forwarda) Draw a detailed mechanism for the reaction and show the structures of the endo and exo products.b) Label the endo and exo product in part a. c) Which transition state is lower in energy, the one leading to the exo product or the one leading to the endo product? d) Which product do you expect to be more stable (the exo or the endo product) and why? Using your answers to part c and d, draw the reaction co-ordinate diagram for the Diels-Alder reaction shown above. Which product do you expect to be favored at high temperatures, and which product at low temperatures (which product is the kinetic product and which one the thermodynamic product)?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY