
Concept explainers
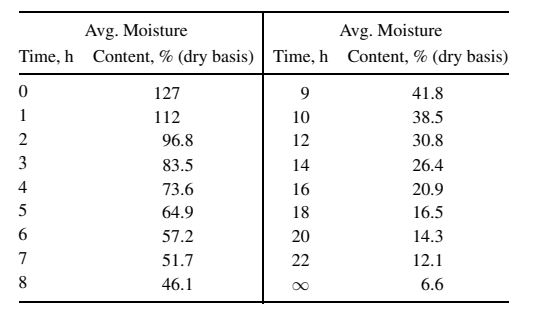
Gilliland and Sherwood obtained data for the drying of a water-wet piece of hemlock wood measuring 15.15x14.8x0.75 cm, where only the two largest faces were exposed to drying air, which was at 25oC and passed over the faces at 3.7 m/s. The wetbulb temperature of the air was 17oC and pressure was 1 atm. The data below were obtained for average moisture content (dry basis) of the wood as a function of time. From these data, determine whether Case 1 or Case 2 for the diffusion of moisture in solids applies. If Case 1 is chosen, determine the effective diffusivity; if Case 2, determine: (a) drying rate in g/h-cm2 for the constant-rate period, assuming a wood density of 0.5 g/cm3 (dry basis) and no shrinkage upon drying; (b) critical moisture content; (c) predicted parabolic moisture-content profile at the beginning of the fallingrate period; (d) effective diffusivity during the falling-rate period. In addition, for either case, describe what else could be determined from the data and explain how it could be verified
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 13 images

- A furnace of 70- by 60- by 90-cm inside dimensions is constructed of a material having athermal conductivity of 1.05 kJ/kg °C. The wall thickness is 15 cm. The inner and outersurface temperatures are 500 and 100 °C, respectively. Calculate the heat loss through thefurnace wall.arrow_forward7. A dryer is shaped like a long semicylindrical duct of diameter 1.5 m. The base of the dryer is occupied with water-soaked materials to be dried. The base is maintained at a temperature of 370 K, while the dome of the dryer is maintained at 1000 K. If both surfaces behave as blackbodies, determine the drying rate per unit length experienced by the wet materials.arrow_forwardExplain the difference (in concept or definition) between boundary layer thickness and thermal boundary layer thickness?arrow_forward
- A large slab of copper is initially at a uniform temperature of 90◦C. Its surfacetemperature is suddenly lowered to 30◦C. Calculate the heat-transfer rate through aplane 7.5 cm from the surface 10 s after the surface temperature is lowered.arrow_forwardA moist cement omnes diameter 10 ohm circular cross-section will be dried using air in a tray and, for example, the variation of moisture content over time will be examined. The heat transfer coefficient was calculated as 0.68 cal/min.cm³.C in the fixed still drying arab. Dry and wet chamber temperatures of the air used for drying are 35 and 20°C, respectively. For example, calculate the mass transfer coefficient during the 10-minute constant speed drying interval. (Note: the humidity of the air (Ha) should be calculated with the humidity diagram.) Data Evaporation enthalpy of water: 2454.2 kj/kg at 20°C 2418.6 k/kg at 35C Vapor pressure of water: 17.54 mm Hg at 20°C. 35C is 42.18 mm Hg.arrow_forwardLayers %R AT Surface Ts 1. Inside air film 20 0.037 2. hardboard, medium density 25mm 0.073 3. concrete blocks, rectangular core:lightweight aggregate 2 core 200mm 0.118 4. expanded poystyrene molded beads 100mm 0.764 5. Outside air film in vented cavity 0.009 What is the AT of the above shown wall for layer 4 (expanded polystyrene molded beads 100mm)? The inside air temperature is +20°C, and the outside air temperature is -4°C. The units for your answer are °C, and your answer is correct if it is within 10% of the correct answer.arrow_forward
- Produce a plot (graph) showing the shape of the PVT-surface for the ideal gas equation.arrow_forward1. A layered wall consists of a copper plate with a thickness of 2.5 cm, the second layer is made of asbestos, the thickness is 3.2 mm, and the third layer is made of glass fiber with a thickness of 5 cm. The overall temperature difference of this wall is 560 ° C. a. Calculate the overall heat transfer rate on this layered wall. b. Calculate T2 and T3arrow_forwardA 4mx5m wall consists of 3 glass windows of 1.5mx1.0m dimensions. The wall has thickness of 0.125 m and a thermal conductivity of 0.5 W/mK, while the glass windows are 6 mm thick with a thermal conductivity of 1.24 W/mK. The values of Internal and external surface conductance for the wall (including glass) are 8.3 W/m K and 34.4 W/m²K, respectively. The internal and external temperatures are 21°C and -30°C, respectively. Calculate the total heat transfer rate through the wall. What percentage of this heat transfer is through the windows?arrow_forward
- The following data were obtained in a drying test of a solid food placed in a laboratory tray dryer. Find the critical moisture content, the critical drying rate, the drying time in the constant-rate period, the time necessary for the moisture to fall from the critical moisture content to 70% (wet basis), and the amount of water that was removed from the product in the drying test. It is given that the initial moisture content is 85% wet basis, the bulk density of the solid is 50 kg dry solids /m², and the thickness of the solid in the tray is 3 cm. Time Weight (kg) (h) 0 3.000 0.5 2.500 1 2.000 Exercises 265 (continued) Time Weight (kg) (h) 2 1.600 3 1.500 4 1.435 6 1.420 8 1.410 1.405 1.400 1.400 10 12 13arrow_forwardExplain graphite block heat exchanger with sketcharrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





