Question
Please help!
1. image
Determine the average background radiation.
2. Image
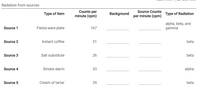
Which is most radioactive?

Transcribed Image Text:Background Radiation
Counts
First minute
15
Second minute
18
Third minute
19
Total counts
Average counts
Transcribed Image Text:ew
Radiation from sources
Counts per
Source Counts
Type of Item
Background
Type of Radiation
minute (cpm)
per minute (cpm)
alpha, beta, and
Source 1
Fiesta-ware plate
167
gamma
Source 2
Instant coffee
31
beta
Source 3
Salt substitute
26
beta
Source 4
Smoke alarm
33
alpha
Source 5
Cream of tartar
35
beta
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Please help I badly need help. ASAP What is (a) an alpha particle? (b) beta particle? (c) a gamma ray? (d) an x ray? (e) a daughter nucleus?arrow_forwardWhich of the following describes safeguards used in nuclear power plants to protect the environment? I. Radioactive sections of the plant are cleaned every week. II. Cooling the reactor prevents an explosion and leak of radiation. III. A mixture of different forms of uranium prevents explosions. O l only Ol and II O Il and III O , II, and IIIarrow_forward3. List and describe the major types of ionizing radiation that are products of nuclear decay and then describe two practical applications of them.arrow_forward
- 8) You start with a 2.00 kg sample of radioactive isotope Y. After 35.0 days, 0.200 kg of the isotope remains. What is the half-life of isotope Y (in days)? Show your work.arrow_forward3. What is the 'best' radiation detecting device that you should use in finding a lost radioactive source that both emits ß and y-radiation? Justify your answer.arrow_forward2) Calculate the density of the nucleus if it had a radius of 10 cm.arrow_forward
- 1. Determine the element created, and its atomic number and mass number when Lead-204 undergoes alpha decay, producing a stable isotope. Write the nuclear reaction equation for this alpha decay. lead 204 => 204 82 Pbarrow_forward11. What type or types of nuclear radiation changes the total number of nucleons in the nucleus? 12. The energy states of electrons in the Bohr Model of the hydrogen atom are negative values. Why is this? What is the meaning of the energy being negative?arrow_forward9. How have uranium mining and extraction facilities affected communities? 10. What kind of radiation is classified as ionizing radiation? Pls answer both of the questions i dont care about bartleby rules i will rate u helpful if u answer both in handwritten cursive answer No plagiarism ill doublecheckarrow_forward
arrow_back_ios
arrow_forward_ios