
Concept explainers
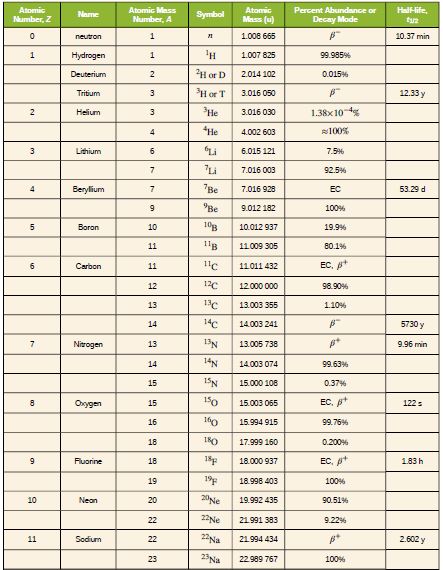
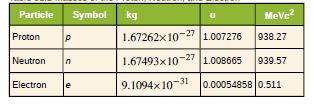
The purpose of this problem is to show in three ways that
the binding energy of the electron in a hydrogen atom is
negligible compared with the masses of the proton and
electron. (a) Calculate the mass equivalent in u of the 13.6-eV
binding energy of an electron in a hydrogen atom, and
compare this with the mass of the hydrogen atom obtained
from list given. (b) Subtract the mass of the proton given from the mass of the hydrogen atom given . You will find the difference is equal to the
electron’s mass to three digits, implying the binding energy is
small in comparison. (c) Take the ratio of the binding energy
of the electron (13.6 eV) to the energy equivalent of the
electron’s mass (0.511 MeV). (d) Discuss how your answers
confirm the stated purpose of this problem.
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

- Some satellites use nuclear power. (a) If such a satellite emits a 2.35 W flux of ? rays having an average energy of 0.440 MeV, how many are emitted per second? (? rays/s) (b) These ? rays affect other satellites. How far away (in km) must another satellite be to only receive one ? ray per second per square meter?arrow_forward(3) A gamma-ray photon with an energy of 1 GeV collides with a stationary proton. The photon scatters backwards, opposite the direction it had before the collision. The proton scatters in the direction that the photon had before the collision. (a) What is the energy of the scattered photon? (b) What is the momentum of the scattered photon? (c) What is the kinetic energy of the scattered proton? (d) What is the momentum of the scattered proton? (e) The Sun generates energy because of nuclear reactions that occur in its core. A sequence of reactionsS that do this is called the PP I chain (pronounced "p p one chain"). In the PP I chain, four protons fuse into a helium-4 nucleus and two positrons, since charge is conserved. Also emitted are two neutrinos, since angular momentum is conserved. The neutrinos have no charge and negligible mass. Assume all motion in the PP I chain is non-relativistic, except for the neutrinos. How many MeV of energy does one PP I chain generate? Include the…arrow_forwardCompare the gravitational potential energy, mgh, exerted on an electron 100 m above theearths surface to the potential energy exerted on an electron by a hydrogen nucleus at adistance of 1 Angstrom. What does this imply?arrow_forward
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





