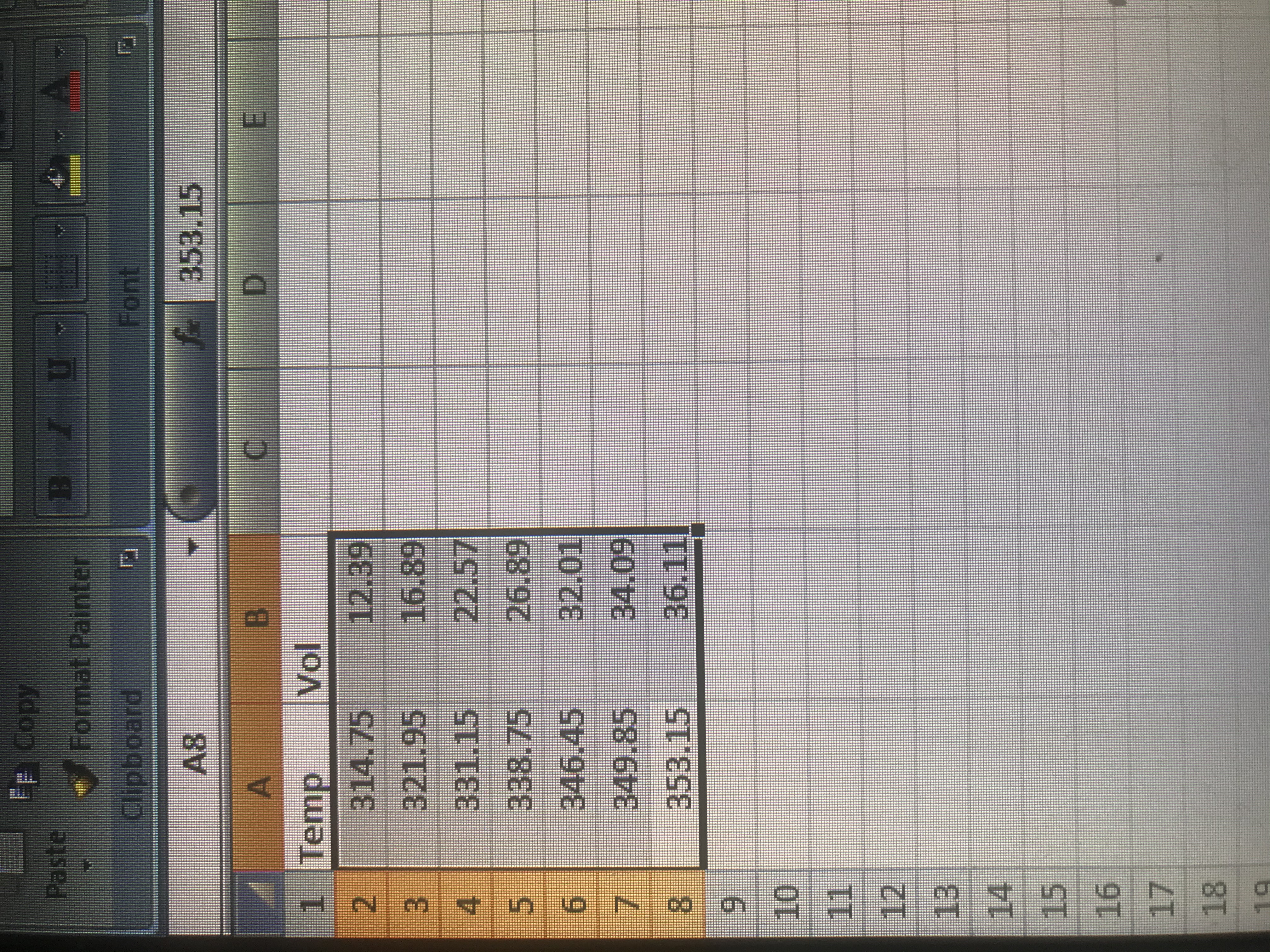
Question
At what temperatures will the air have a volume of 165.0 ml and 75 ml respectively? I have included a picture of the temperatures and volumes from my experiment.
We also had to create a graph of volume in ml (y-axis) versus the temperature in K (x-axis). We are to include the slope and intercept and upload the graph. Could you help me? I'm not sure if my graph is correct or not. Thank you!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- Explain the difference between a rate of change that is positive and one that is negative. Give an example of each. O Consider the ordered pair (x, y) where x is the independent variable and y is the dependent variable. If y increases with the increase in x, then the rate of change of y is positive. Whereas, if y decreases as x increases, then the rate of change is negative. For example, if you consider the speed of a car (y) over time (x), as the car accelerates, the speed increases with increasing time, and the rate of change of its speed is positive. When the car brakes, the rate of change of its speed is negative, because the speed decreases over time. Consider the ordered pair (x, y) where x is the independent variable and y is the dependent variable. If y increases with a decrease in x, then the rate of change of y is positive. Whereas, if y decreases as x decreases, then the rate of change is negative. For example, if you consider the speed of a car (y) over time (x), as the car…arrow_forwardA cylinder containing 100.9 cubic centimeter of gas at a pressure of 376 kPa when its temperature is 534 K. Given that its temperature is unchanged when the pressure was increased by a factor of 5.9, Determine the new volume of the gas (In cubic centimeter). Note: Your answer must be in cubic centimeter, however, do not include the unit, just enter the magnitude that corresponds to the final volume in cubic centimeter. Round your answer to 2 decimal points Round your answer to 2 decimal pointsarrow_forwardPlease help. This problem involves finding the radius and volume of a platinum atom using its density. Thank you.arrow_forward
- For this problem, we want to estimate the answer, so our assumptions may be a little unrealistic. Suppose we want to estimate how much air we need to send to a space station, assuming we cannot recycle air. Suppose four astronauts are in a spherical space station. If each of them typically breathes about 500 cm³of air with each breathe, and take 15 breathes per minute (average resting value): a. What is the volume of air you would need in the space station if these four astronauts stayed for a full year? b. If the density of air is 1.25 kg/m³ and it costs (thanks to SpaceX) a mere $100/kg to send objects to the space station, how much money would the astronaut air supply cost?arrow_forwardIn a hypothetical diesel engine the fuel is compressed from 528mL to 39.7 mL. This rasies the temperature of the fuel form 25°C to its ignition temperature of 210°C. If the fuel begins at 1.00atm, what is the pressure of the fuel at its ignition temperature.? Express your answer to 1 decimal place. Be sure to use the proper abbreviation of the UNITSarrow_forwardNeeds Complete typed solution with 100 % accuracy.arrow_forward
- A cylinder containing 239.6 cubic centimeter of gas at a pressure of 342 kPa when its temperature is 406 K. Given that its volume is unchanged when the pressure was increased by a factor of 1.2, Determine the new Temperature of the gas (In Kelvin). Note: Your answer must be in Kelvin, however, do not include the unit, just enter the magnitude that corresponds to the final volume in Kelvin. Round your answer to 2 decimal points Round your answer to 2 decimal pointsarrow_forwardA. On the average, what volume of blood, in liters, does the heart pump during each beat? B. On the average, what volume of blood, in cubic centimeters, does the heart pump during each beat?arrow_forward8.36 grams of a mystery substance of food with a molar mass of 149.36 g/mol is burned in a bomb calorimeter. The water in the bomb calorimeter absorbs 105,961 Joules from the burning of the substance. Calculate the heat of combustion of the mystery substance in kJ/mol. (Please input your answer as a negative number.) (DO NOT PUT UNITS IN YOUR ANSWER.) Assume that all of the heat lost by the substance is transferred to the water and no heat is lost to the surroundings.arrow_forward
- A hot tub with a surface area of 28 ft2 is filled with water to a depth of 29 in . Hint: volume is calculated as area × height (A × h). A) What is the volume of water in the tub, in liters? Express your answer to two significant figures and include the appropriate units. B) How many kilojoules are needed to heat the water from 59∘F to 103 ∘F? Express your answer to two significant figures and include the appropriate units. C) If the hot-tub heater provides 5900 kJ/min, how long, in hours, will it take to heat the water in the hot tub from 59∘F to 103∘F? Express your answer to two significant figures and include the appropriate units.arrow_forwardThe volume of air in one beach ball is 972? in3. What is the volume of air in a ball whose radius is 3 inches greater than the first beach ball? A beach ball is spherical in shape. Recall that the formula for the volume V of a sphere where r is the radius is given by the following formula. V=4/3?r3 It is given that the volume of the first beach ball is 972? in3. Substitute this value for V in the volume formula for a sphere. ______= 4/3?r3arrow_forward
arrow_back_ios
arrow_forward_ios