
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
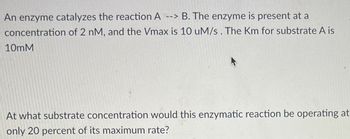
Transcribed Image Text:An enzyme catalyzes the reaction A --> B. The enzyme is present at a
concentration of 2 nM, and the Vmax is 10 uM/s. The Km for substrate A is
10mM
At what substrate concentration would this enzymatic reaction be operating at
only 20 percent of its maximum rate?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Sketch a graph showing how the concentration of product P varies with time for chymotrypsin-catalysed hydrolysis using a substrate that displays both Michaelis-Menten kinetics and burst kinetics. On the same graph show the effect of doubling the enzyme concentration, keeping all other conditions the same.arrow_forwardWhat is the difference between substrate-level phosphorylation and oxidative phosphorylation? Please give an example of each.arrow_forwardWhy do the cytochrome electron-transfer processes have different standard reduction potentials, despite the fact that they all utilize the identical iron oxidation–reduction reaction?arrow_forward
- One of the hallmarks of competitive inhibition is that there is constant competition betweenthe substrate and the inhibitor for binding to the enzyme active site.a) If [inhibitor] >> [substrate], which compound “wins” (i.e., occupies the active site a greaterpercentage of the time)?b) If [substrate] >> [inhibitor], which compound “wins” (i.e., occupies the active site a greaterpercentage of the time)?arrow_forwardIn the pathway for degradation of BCAAs, which reaction takes place prior to the action of the BCKDC and what is the essential coenzyme for the reaction?arrow_forwardDescribe the rate of enzyme-catalyzed reaction with increasing substrate concentration at constant enzyme concentration. In what ways does hydrogen ion concentration affect enzyme activity?arrow_forward
- The enzymatic activity of an enzyme with Kg = 2 mM that converts substrate S into product P is measured at an initial substrate concentration S, of 10 µM. After 5 min, the substrate concentration is halved. What is the rate constant k, the maximal velocity vmax and the concentration of product after 12 min?arrow_forwarda) Based on the data shown in the image, what are the Km and Vmax for the enzyme with L-DOPA and D-DOPA? Show any relevant analyses or calculatins you did to determine these values. ( HINT a graph might be helpful here! ) b) Based on your answer to part a, briefly describe how the kinetics of the enzyme differs for the two substrates. Which Substrate has better binding affinity to the enzymearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON