
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
At the pressure of P=0.1MPa, the saturation temperature of water is Tsat=100 ºC, the specific volume of the saturated liquid water is vf=0.001043 m3/kg, and the specific volume of the saturated vapor water is vg=1.6720 m3/kg.
(3) Please match regions shown in the diagram with correct names.
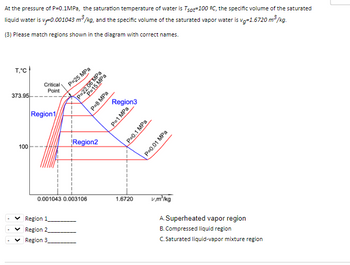
Transcribed Image Text:At the pressure of P=0.1MPa, the saturation temperature of water is Tsot-100 °C, the specific volume of the saturated
liquid water is v. 0.001043 m³/kg, and the specific volume of the saturated vapor water is vg=1.6720 m³/kg.
(3) Please match regions shown in the diagram with correct names.
T,°C
373.95
100
Critical
Point
Region1
P=25 MPa
Region 1
Region 2
Region 3
1
Region2
0.001043 0.003106
P=22.06 MPa
P=15 MPa
P=8 MPa
Region3
P=1 MPa
1.6720
P=0.1 MPa
P=0.01 MPa
v.m³/kg
A. Superheated vapor region
B. Compressed liquid region
C. Saturated liquid-vapor mixture region
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- An oxygen tank has a volume of V = 3.00 m3. The gage pressure of oxygen in the tank is Pgage = 400.00 kPa. The room temperature is T = 27.00 ˚C and the atmospheric pressure is Patm = 100.00 kpa. The gas constant of oxygen is R = 0.25980 kPa/(kg·K). Oxygen can be treated as an ideal gas. Determine (1) the specific volume of oxygen, v =_____ m3arrow_forwardEthylene is heated at a constant pressure of 5 MPa and 20 degrees Celsius until the temperature reaches 200°C. Using the generalized compressibility diagram, determine the specific volume change in ethylene. FOLLOW THE NEXT STEPS Step 1. Sketch/Paraphrase (Draw the sketch and process diagram if necessary) 2. Theoretical Concepts / Formulas (write down the concepts you are applying and theformulation that will help you solve the problem 3. Information (tables, data in the program, graphs) Assumptions (In the event that they arenecessary) 4.Development Solution (Pay special attention to the units shown and requested)arrow_forwardIn an electrolysis of water experiment, 39.75 mL of H2 gas was collected over water at 60.0 °C and an atmospheric pressure of 1.03 atm. The vapour pressure of water at 60.0 °C is 19.92 kPa. What mass of H2 was collected?arrow_forward
- 1.00×104 cm³ of 200 °C steam at a pressure of 10.0 atm is cooled until the volume of the liquid water? condenses. What is Part A Give your answer in cm³. Express your answer with the appropriate units.arrow_forwardAn oxygen tank has a volume of V = 3.00 m3. The gage pressure of oxygen in the tank is Pgage = 400.00 kPa. The room temperature is T = 27.00 ˚C and the atmospheric pressure is Patm = 100.00 kpa. The gas constant of oxygen is R = 0.25980 kPa/(kg·K). Oxygen can be treated as an ideal gas. Determine (2) the mass of oxygen, m =_____ kgarrow_forwardWhat is the pressure on a pure water surface if it starts to boil at a temperature of 110C. (Answer 1777.2mb)Show every steparrow_forward
- please help me with thisarrow_forwardA real gas exists at 130 C and 1.2 MPa. It is known that the critical temperature and critical pressure of the gas is 374.2 k and 4.059 MPa. It is also know that the ideal gas constant (R) of the gas is 0.12 kPa-m/ S/kg-K. In the question that follows, select the answer that is closest to the true value. Use the compressibility factor (Z) to determine the specific volume in units of m/kg. ,3, Tuesday Dece mber 7 2021 5:52:58 DM CSTarrow_forwardA piston-cylinder device containing 2kg of saturated H20 at 187 °C has initial specific volume of 0.13 kg/m³. The H20 is then expanded to a pressure of 300 kPa and a temperature of 187 °C. Fill in the following chart of properties for each state, and plot the two states on the given graph. Show all your work on this sheet or the next. State 1 Property P (kPa) State 2 300 T (°C) v (m³/kg) u (kJ/kg) x (phase or quality) 187 187 0.13 Varrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY