
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
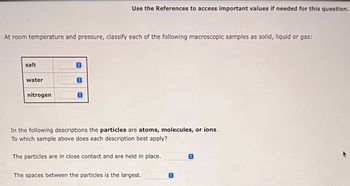
Transcribed Image Text:At room temperature and pressure, classify each of the following macroscopic samples as solid, liquid or gas:
salt
water
nitrogen
O
Use the References to access important values if needed for this question.
0
In the following descriptions the particles are atoms, molecules, or ions.
To which sample above does each description best apply?
The particles are in close contact and are held in place.
The spaces between the particles is the largest.
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the correct answer?arrow_forwardComplete each of the following sentences by placing the word chemical or physical in the blank: The crushing of rocks to make gravel is a procedure. The burning of wood is a process. Converting water into hydrogen and oxygen is a procedure. The freezing of a liquid is a process.arrow_forwardProvide mechanism (arrow pushing) for this Friedel Crafts alkylation, include electrophile and resonance structures Benzene Cat. H2SO4arrow_forward
- Hydrogen peroxide (H2O2) is a mild antiseptic used to prevent infection in minor cuts and scrapes. The density of H2O2 is 1.45 g/mL. The cylinder container that the hydrogen peroxide is stored in has a radius of 8.5 cm and a height of 22 cm. If the container is full, calculate the number of oxygen atoms in the container. (Vcylinder = πr2h).arrow_forwardA hypothetical metal has a theoretical density of 11.47 g/cm3. This metal adopts a cubic crystal structure with an edge length of 0.387 nm and atomic radius of 0.137 nm. Find the atomic weight of this metal in g/mol. NA = 6.022 x 1023 atoms/mol. Express your answer in two decimal places only. Do not put the units.arrow_forwardHow many grams of oxygen (O) are present in a 6.41 g sample of potassium nitrate (KNO3)? Enter your answer in decimal form with the correct number of sig figs. Use the proper abbreviation for the units. NOTE: If you have taken chemistry before and know what diatomic means, do not take that into consideration. Use just O for this. There are reasons for this.arrow_forward
- Tia has a sample of pure gold (Au). She weighed the sample and the result was 84.784.7 grams. Tia wants to determine the number of atoms in the sample. Calculate the number of atoms in 84.784.7 g of pure gold.arrow_forwardAn analytical chemist measures the amount of Elements E₁ and E₂ in four samples of an unknown Substance. sample of E1 1 2 3 mass mass of E2 4 16.0 g 9.1 g 17.1 g 9.8 g 18.5 g 10.6 g 12.0 g 6.9 g It's known that X contains no elements other than E₁ and E₂. Using this information, answer the questions in the table below. Is X a pure substance or a mixture? If you don't have enough information to decide, choose can't decide. If you said X is a pure substance, calculate the mass of Element E₁ the analytical chemist would find in a new 10.0 g sample of X. Round your answer to 2 significant digits. O pure substance O mixture O (can't decide) garrow_forwardWhich statement is FALSE? O A pure substance can be varied by changing the proportion of pure substances making it up A mixture can be separated into 2 or more substances by physical or mechanical means A pure substance had properties that are constant, no matter where you find it A pure substance has constant chemical composition A mixture's composition can be varied by changing the proportion of pure substances making it uparrow_forward
- How many moles are there in 1.0 g of the element Al? Your answer must contain the correct number of significant figures. 13 6.022 x 10^23 0.037 4 x 10^-2 1.0arrow_forwardCalculate the number of boron atoms in a 120.0 g sample of tetraborane (B4H10). Be sure your answer has a unit symbol if necessary, and round it to 4 significant digits. 0 ☐x10 X Śarrow_forwardThe radius of a potassium atom is 231 pm. How many potassium atoms would have to be laid side by side to span a distance of 4.87 mm? atomsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY