
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
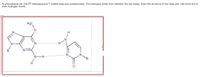
Transcribed Image Text:At physiological pH, the 06-methylguanine:T wobble base pair predominates. Two hydrogen bonds form between the two bases. Draw the structure of this base pair. Use bond Any to
draw hydrogen bonds.
H;C
H
N-
N-
R
-N.
N-H-
R
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Select all of the true statements regarding the molecule below: Thymine 5' end Adenine 3' end OH NH2 NHN Phosphate- deoxyribose backbone 0H2N H2N NH2 o. Guanine Cytosined 3' end 5' end This is RNA O This is DNA O This is a monomer O The backbone of these nucleotides is a 5-carbon sugar. This nucleic acid is found in healthy human cells. This nucleic acid is the hereditary material for coronaviruses. This type of nucleic acid is usually found as a single stranded molecule. This nucleic acid is the hereditary material for some viruses, but not the SARS-CoV- 2 virus.arrow_forwardConsider the protein below: HO HỘ NH CH-OH CH Identify/Name the noncovalent interaction between groups in the following locations: Σarrow_forwardA Uracil B Adenine HN Cytosine Guanine 2 H₂N-C 6 3 N Which of the following nitrogenous bases is represented by this structure? 5 C C N 7 IZⓇ 8 CHarrow_forward
- Describe why G-C bonding is stronger than A-T. Why is it important?arrow_forwardName the nitrogenous bases that are bondedarrow_forwardDraw the structure of histidylthreonine, a dipeptide made from histidine and threonine, as it would appear at physiological pH. Explanation Click and drag to start drawing a structure. Chock 0, X 0 :0 E Ahmarrow_forward
- Why is the peptide bond so stable in water?arrow_forwardWhat would the net charge be of a polypeptide with the sequence M-D-R-N-Q-K-R-W at pH 8? hint ionizable groups include N-terminus (pKa = 9.0), D (pKa = 3.9), R (pKa = 12.5), K (pКa %3D 10.5) O +2 O-1 O +1arrow_forwardWhich one bracketed region most likely illustrates a phosphodiester bond in Figure 1? The bracketed region marked ‘i’, from phosphate group to phosphate group. The bracketed region marked ‘j’, from methyl group to methyl group. The bracketed region marked ‘k’, from 3’ carbon to 5’ carbon. None of the above and I have explained my reasoning here.arrow_forward
- Draw the two dipeptides possible when glutamine and cysteine undergo dehydration to form a peptide bond. Draw the dipeptides in zwitterionic form.arrow_forwardIs serine chiral? Draw serine and identify the chiral atom. Explain why serine is chiral.arrow_forwardcis, cis-9,12-OCTAdecadiENoic acid Enumerate the number of carbons and unsaturations (double bonds) in this moleculearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON