
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
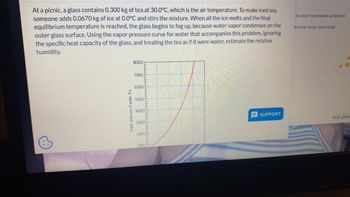
Transcribed Image Text:At a picnic, a glass contains 0.300 kg of tea at 30.0°C, which is the air temperature. To make iced tea,
someone adds 0.0670 kg of ice at 0.0°C and stirs the mixture. When all the ice melts and the final
equilibrium temperature is reached, the glass begins to fog up, because water vapor condenses on the
outer glass surface. Using the vapor pressure curve for water that accompanies this problem, ignoring
the specific heat capacity of the glass, and treating the tea as if it were water, estimate the relative
humidity.
in your homework problem!
minute-keep searching!
Vapor pressure of water, Pa
8000
7000
6000
5000
4000
SUPPORT
3000
and glass
2000
1000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A mountain hiker tightens the cap on a half-full plastic water bottle at a high altitude. The volume of air in the sealed bottle is 5 x 10-4 m³, the pressure is 64 kPa and the temperature is -8 °C. The hiker descends to a lower altitude where the pressure is 94.2 kPa and the temperature is 11 °C. What is the final volume of the sealed air inside the bottle? (this is what causes the plastic bottle to deform if you've ever experience this situation)arrow_forwardA bubble having a diameter of 27 cm is re- leased from a depth of 4.6 m from the bottom of a swimming pool. The temperature of the water at the surface is 18°C, whereas it is 17°C at the point of release. What will the diameter of the bubble be when it reaches the surface? The acceleration of gravity is 9.8 m/s² and the density of water is 1000 kg/m³. Answer in units of cm. Answer in units of cm.arrow_forwardWhat is the density of water vapor in the air on a hot, dry day in the desert when the temperature is 40.0 °C and the relative humidity is 5.00 %? The vapor pressure of water at 40.0 °C is Py = 7.34 x 10° Pa and the molecular mass of water is M = 18.0 g/mol. Enter your answer in units of grams per cubic meter. density: g/m3arrow_forward
- A student wakes up late on a cool spring morning and realizes they are late for Physics class. They run to their car, start it, and begin driving to school immediately. Before the car is driven, the (absolute) tire pressure is 475.0 kPa and the air temperature is 280.1 K. As the car is driven down the road, the tires heat up and by the time the student reaches the parking lot, the temperature of the air inside the tires is 290.3 K. Assuming that the volume of the tires does not change, what is the pressure in the tires when the student reaches the parking lot? Give your answer in kPa.arrow_forwardTwo 39.5-g ice cubes initially at 0°C are added to 460 g of water at 22.0°C. Assuming this system is insulated and ignoring heat transfer with the glass, what is the equilibrium temperature of the mixture? °Carrow_forwardA cylindrical glass flask with a diameter of 8 cm and the height of 20 cm is fully filled with water at 20°C. When the temperature of the water (and consequently the inner surface of the flask) is raised to 100° C, determine the volume of the water that overflows? HINT 1: Don't forget to calculate the initial volume of the water and flask HINT 2: The volume expansion coefficient for ordinary glass and water can be found in the table of Week 4 (Slide #20). HINT 3: Calculate the volume expansions for both water and the flask HINT 4: The difference between the final volume of water and the final volume of the flask is the amount of overflow. PLEASE UPLOAD YOUR HANDWRITTEN SOLUTION BY CLICKING ON THE "ADD A FILE" BUTTON.arrow_forward
- The boiling point of a liquid is 28.3°C. A portion of the liquid is at equilibrium with its vapor at 22.3°C in a sealed flask. The temperature rises to to 32.6°C.What happens inside the container? Liquid is converted to vapor, the new equilbrium mixture contains less liquid and more vapor. The vapor is all converted to liquid. Vapor is converted to liquid, the new equilibrium mixture contains more liquid and less vapor. The liquid is all converted to vapor.arrow_forwardA bubble having a diameter of 62 cm is re- leased from a depth of 4.6 m from the bottom of a swimming pool. The temperature of the water at the surface is 21°C, whereas it is 15°C at the point of release. What will the diameter of the bubble be when it reaches the surface? The acceleration of gravity is 9.8 m/s² and the density of water is 1000 kg/m³. Answer in units of cm.arrow_forwardA 4.5-cm-diameter, 0.50-mm-thick spherical plastic shell holds carbon dioxide at 2.0 atm pressure and 25°C. CO2 molecules diffuse out of the shell into the surrounding air, where the carbon dioxide concentration is essentially zero. The diffusion coefficient of carbon dioxide in the plastic is 2.5 × 10-¹² m²/s.arrow_forward
- A hot air balloon uses the principle of buoyancy to create lift. By making the air inside the balloon less dense then the surrounding air, the balloon is able to lift objects many times its own weight. A large hot air balloon has a maximum balloon volume of 2090 m3. a. If the air temperature in the balloon is 54 °C, how much additional mass, in kilograms, can the balloon lift? Assume the molar mass of air is 28.97 g/mol, the air density is 1.20 kg/m3, and the air pressure is 1 atm.arrow_forwardAn apartment has the dimensions 16 m by 8 m by 5 m. The temperature is 30°C, and the relative humidity is 51 percent. What is the total mass (in kg) of water vapor in the air in the apartment?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON